5 Secret Health Benefits Of Gardening Working In Dirt Boosts Mood, Lowers Dementia Risk Kim Hayes

Article Menu
/ajax/scifeed/subscribe
Open Access Review
From Obesity to Hippocampal Neurodegeneration: Pathogenesis and Non-Pharmacological Interventions
Department of Rehabilitation Sciences, Faculty of Health and Social Sciences, Hong Kong Polytechnic University, Hung Hom, Hong Kong
*
Author to whom correspondence should be addressed.
Received: 29 October 2020 / Revised: 21 December 2020 / Accepted: 23 December 2020 / Published: 28 December 2020
Abstract
High-caloric diet and physical inactivity predispose individuals to obesity and diabetes, which are risk factors of hippocampal neurodegeneration and cognitive deficits. Along with the adipose-hippocampus crosstalk, chronically inflamed adipose tissue secretes inflammatory cytokine could trigger neuroinflammatory responses in the hippocampus, and in turn, impairs hippocampal neuroplasticity under obese and diabetic conditions. Hence, caloric restriction and physical exercise are critical non-pharmacological interventions to halt the pathogenesis from obesity to hippocampal neurodegeneration. In response to physical exercise, peripheral organs, including the adipose tissue, skeletal muscles, and liver, can secret numerous exerkines, which bring beneficial effects to metabolic and brain health. In this review, we summarized how chronic inflammation in adipose tissue could trigger neuroinflammation and hippocampal impairment, which potentially contribute to cognitive deficits in obese and diabetic conditions. We also discussed the potential mechanisms underlying the neurotrophic and neuroprotective effects of caloric restriction and physical exercise by counteracting neuroinflammation, plasticity deficits, and cognitive impairments. This review provides timely insights into how chronic metabolic disorders, like obesity, could impair brain health and cognitive functions in later life.
1. Introduction
Western dietary patterns and sedentary lifestyle have fueled the current obesity epidemic [1]. Processed and refined food is constituted by saturated fats, added sugar, and salts, which contribute to high caloric intake under chronic consumption. Besides, the primary consumption of red meats and dairy products with a lack of vegetables and fresh fruits are the other characteristics of the Western dietary pattern. However, when caloric consumption far exceeds expenditure under prolonged physical inactivity, metabolic syndromes are developed. Metabolic syndrome encompasses a cluster of risk factors that could lead to cardiovascular diseases and diabetes [2]. Criteria for clinical diagnosis of metabolic syndrome include increases in waist circumference, triglycerides, blood pressure, fasting glucose, and a reduction in high-density lipoprotein cholesterol [3]. A recent review has summarized that the key pro-inflammatory constituents from the dietary sources, including saturated fatty acids, cholesterol, added sugars, refined grains, purines, dietary carnitine, and dietary histidine [4]. Chronic and excessive dietary intake of these constituents may trigger a chronic inflammatory response in multiple tissue organs, which then develop into non-communicable diseases [4]. Both diabetes and dementia are examples of non-communicable diseases. It is well-known that the Western dietary pattern is strongly linked to the development of obesity and type 2 diabetes mellitus (T2DM) [5]. Other studies also report the association between adopting a Western dietary pattern and impairments in hippocampal-dependent learning and memory performance across the lifespan [6,7,8,9,10,11,12]. A clinical study demonstrates that a causative relationship by subjecting a group of healthy young adults (n = 102) to a short exposure (four days) to a high caloric diet. Healthy young adults are subjected to high saturated fat and added sugar breakfasts (53% total fats, 37.5% carbohydrates, 11.5% proteins) or a control diet (15.9% total fat, 31.8% carbohydrates, 51.3% proteins) for four days [13]. Four days after, individuals consuming a high caloric diet have lower retention scores in the Hopkins-Verbal Learning Test as compared to the controls, implicating a decline in hippocampal-dependent learning and memory performance. Of particular note, a negative correlation between retention score and blood glucose level is observed. Moreover, interoceptive sensitivity to satiety is also reduced in these individuals consuming high caloric diet, suggesting a lower appetitive control. Another clinical study further demonstrates a correlation between impaired appetitive control and decline in memory retention test score after one week of high caloric diet consumption (n = 110) [14]. The enigmatic relationship underlying high-caloric diet, obesity, diabetes, and hippocampal plasticity has been actively investigated in rodent studies.
Obesity could predispose individual to type 2 diabetes mellitus (T2DM), which is characterized by hyperglycemia and insulin resistance. Both peripheral [15,16] and central insulin resistance [17,18] are associated with cognitive impairment and neurodegenerative diseases. As supported by animal studies, progressive peripheral and central insulin resistance are linked to Alzheimer's disease (AD) pathology and cognitive impairments [19,20,21,22,23,24]. Also, the AD-associated insulin signalling impairment in the brain resembles the dysfunction of insulin signalling in the peripheral organs, including the pancreas, adipose tissues, liver, and muscle, in diabetes [25,26,27,28,29]. β-amyloid accumulation is one of the pathologies found in the AD brains. In the form of amylin or islet amyloid polypeptide accumulation, amyloidosis is also observed in the pancreas under diabetic condition [30]. Notably, AD is classified as type 3 diabetes presenting impaired insulin signalling and neurological deficits in the brain [31]. It has been previously reported that adipokines secreted from the adipose tissue could influence brain plasticity and cognitive function in both physiological and pathological conditions, respectively [32,33]. Indeed, literature has reported that individuals with obese [34,35,36] and diabetes [37,38] could have a higher incidence of dementia, suggesting that early-life metabolic syndrome could contribute to dementia in the later life.
Amble animal studies have shown that diabetic conditions are associated with insulin resistance and cognitive impairments [39,40,41,42]. Chronic consumption of high-fat diet impairs spatial learning and memory performance in the radial arm maze in juvenile mice [43]. Leptin receptor deficiency also impairs spatial memory in the Morris water maze task [44] and working memory in the Y-maze task, as well as disrupts prepulse inhibition in db/db mice [45]. The hippocampus plays an essential role in regulating spatial learning and memory processes, as well as affective behaviours [46]. Specifically, the dorsal hippocampus is involved in spatial learning and memory, while the ventral hippocampus is involved in mood regulation [47]. Conversely, impaired hippocampal plasticity by stress, ageing, or metabolic disorders could lead to learning and memory deficits, mood disorders, and eating disorders [48,49,50,51,52]. Diabetes-induced structural and synaptic deficits in the hippocampus are predisposing factors of learning and memory impairments and mood disorders [53,54,55,56,57,58,59,60,61]. The dentate gyrus (DG) of the hippocampus is one of the neurogenic zones with continuous generation of new neurons in the adult brains [62]. Animal studies have demonstrated that obese and diabetes suppress cell proliferation [58,63,64,65], neuronal differentiation [43,65,66], and cell survival [51] of the new-born cells in the hippocampal DG. Furthermore, obese and diabetic conditions also reduce dendritic complexity [44,67] and synaptogenesis [44,51] in the hippocampus, suggesting the hippocampus is highly vulnerable brain regions to metabolic dysregulation.
Long-term potentiation (LTP) and long-term depression (LTD) are cellular mechanisms underlying changes in synaptic plasticity [68] and learning and memory formation [69]. LTP impairment [44,70,71] and LTD facilitation [72] in the hippocampus are linked to learning and memory deficits in diabetic rodents [73,74,75]. Deficits in LTP aggravate with age [73], disease duration [76], and severity [77] of the diabetic condition. The role of glutamatergic transmission in LTP formation is critical, whereas reducing the extracellular level of glutamate at the synaptic cleft could impair LTP induction [78]. Glutamine-glutamate (Gln/Glu) ratio is an indicator of glutamatergic synaptic transmission with an implication of enhanced glutamatergic transmission in high Gln/Glu ratio [79]. Nevertheless, prolonged high-fat diet (HFD) for ten weeks reduces the Gln/Glu ratio in the hippocampus with substantial elevation in glutamate levels, but not glutamine levels [80]. Glutamate accumulation at the synaptic cleft may lead to excitotoxicity [81]; and thus, impairs LTP formation. Moreover, chronic high-caloric diet reduces protein expression levels of synaptic receptors, which could account for impairment in synaptic transmission in obese and diabetic conditions. To facilitate LTP, GluA1 subunit-containing AMPA receptors are rapidly recruited to the postsynaptic membrane upon stimulation by insulin or by NMDA receptor-mediated calcium influx [82]. Six-week HFD induces GluA1 palmitoylation and reduces phosphorylation, and consequently reduces AMPA receptor function [83], which could be linked to decreased postsynaptic contents of GluA1 and GluA2 in the hippocampus [84]. On the other hand, HFD promotes palmitoylation of NMDA receptor subunits (GluN2A and GluN2B) [83]. Specifically, eight weeks [85], but not six weeks [83] of HFD intake affects GluN2B expression, suggesting the time-dependent effect of HFD on NMDA receptor function. Of note, HFD also reduces the expression levels of PSD-95 (a postsynaptic scaffolding protein in glutamatergic synapses) and gephyrin (a postsynaptic scaffolding protein mediating aggregation of GABAA receptors), suggesting that HFD could impair synaptic plasticity by modulating both glutamatergic and GABAergic function in the hippocampus [86].
Comorbid depression with diabetes and obesity is observed in humans [87,88]. Similarly, concomitant HFD feeding and corticosterone administration accelerate the onset of diabetes in a rat model [89], highlighting the neuroendocrine interaction of stress and high-caloric diet on promoting the pathogenesis of diabetes. Obese and diabetic animals show depression-like behaviors [90,91,92], which could be partly contributed by impaired hippocampal adult neurogenesis [93,94,95]. Both rodent models of depression [96,97,98,99] and diabetes [100,101,102] have shown a hyper-activated hypothalamic-pituitary-adrenal (HPA) axis, resulting in elevated glucocorticoid levels in response to stressors. The hippocampus exerts inhibitory feedback control to the activation of the HPA axis through GABAergic innervation to the paraventricular nucleus (PVN) of the hypothalamus [103,104,105]. Hippocampal dysfunction reduces the capacity to regulate the activity of the HPA axis. Four-week HFD increases serum levels of corticosterone and suppresses hippocampal newborn cell survival [64]. Exogenous administration of corticosterone suppresses hippocampal adult neurogenesis. It impairs dendritic complexity which is linked to increased depression-like behavior and impaired learning and memory [106,107,108], suggesting that high intake of dietary fat could dysregulate hippocampal neurogenesis through hyper-activating the HPA axis.
Recent studies have reported that an HFD feeding protocol as short as one week is sufficient to impair learning and memory performance, whereas hippocampal plasticity impairment and mood disturbances occur as the HFD feeding continues [109,110]. However, one-week HFD induces object recognition deficits, which can be restored by HFD withdrawal [111]. On the other hand, an eight-week dietary reversal from 16-week HFD can also restore learning and memory deficits and glucose intolerance, though insulin resistance is irreversible [112]. Uncontrolled food-seeking behaviors are observed in rodents consuming high-calorie palatable food [113,114] as well as in obese [115] and diabetic rodents [116]. The data collectively suggest that permissive obesogenic environment encourages hyperphagia-like behavior, which further promotes the progression of metabolic and neurocognitive diseases. Overproduction of corticosterone due to hyper-activated HPA axis promotes the secretion of the orexigenic neuropeptide called agouti-related peptide (ARP) in the hypothalamus, and thus promotes food consumption [117,118]. This finding could partly explain clinical observation in which individuals exposed to psychosocial stress and obesogenic environment have increased food consumption [119]. Furthermore, it is known that the hippocampus is involved in memory-related to eating episodes and food cue. For example, inhibiting GABAA receptor in the dorsal hippocampus promotes postprandial sucrose consumption [120], whereas sucrose consumption promotes neuronal activation in the dorsal CA1 sub-region [121]. These findings have suggested that the dorsal hippocampus plays a role in regulating eating behavior [122]. It is also found that food consumption could be increased by manipulating the ventral hippocampus functioning through different approaches including lesion [123], optogenetic inactivation [124], local activation of leptin and GLP-1 receptors [125,126], inhibition of GABAA receptor [127]. In sum, impaired synaptic plasticity in the hippocampus and hyperactivation of the HPA axis can lead to uncontrolled appetitive behaviors, which further aggravate the severity of metabolic syndromes [128,129].
The above studies have suggested the possible effects of obesogenic diet and diabetic condition on impairing neural plasticity in the hippocampus, which in turn could contribute to cognitive decline, emotional disturbances, as well as hyperphagia behaviors. Adipose tissue regulates hippocampal functions via releasing adipokines that can directly or indirectly modulate both neural plasticity [58,130,131,132,133,134,135,136,137,138] and neuroinflammation [44,55,139,140]. However, the potential mechanisms of how adipose tissue inflammation in obesity can progressively lead to neurodegeneration and neuroinflammation in the hippocampus have yet to be explored. In this review, we summarize how adipose tissue dysregulation and inflammation can lead to neurodegeneration and cognitive decline. Leptin resistance and inflammasome activation in the adipose tissue contribute to cognitive deficits, in which neural plasticity is impaired, and neuroinflammatory responses are activated in the hippocampus. Inflammation occurred in the adipose tissue suppresses the secretion of anti-inflammatory adipokines, such as adiponectin [141,142], that can directly affect hippocampal plasticity [132,143,144,145]. Though both impaired neuroplasticity and neuroinflammatory response are presented in diet-induced obese and diabetic models, microglial activation could play a predominant role in impairing hippocampal plasticity and cognitive function in these models. We also explored the possible role of microglia-mediated synaptic stripping in diabetic or obese. While withdrawing obesogenic diet could be one of the non-pharmacological interventions to improve metabolic profiles, physical exercise and balanced diets are known to be effective in ameliorating memory deficit and amyloid deposition in an AD mouse model [146]. Physical exercise is known as a non-pharmacological intervention to combat metabolic syndromes [147] and cognitive deficits [148,149] in obese and diabetic individuals. In response to physical exercise, multiple organs are known to secrete cytokines or metabolic hormones. The term "exerkine" has been used to describe secretory factors-induced by physical exercise acting on the brain and peripheral organs. Some of the exerkines are found to be pro-cognitive; therefore, this review also summarized the roles of some exerkines secreted from the liver, muscle, and adipose tissue on modulating neuronal metabolism, neuroinflammation and hence neuroplasticity that underlying changes in cognitive functions.
2. Gut Is a Potential Origin of Chronic, Low-Grade Inflammation in Obesity and Diabetes
Chronic, low-grade inflammation in the adipose tissue is a characteristic of diet-induced obesity [150]. The critical effector that triggers diet-induced the adipose tissue inflammation is often masked. This is because metabolic and immunological complications have arisen from multiple organs in obesity, or when diabetes is diagnosed. Studies have suggested that the gut microbiome is inextricably linked to obesity. A pioneering animal study has reported that naïve recipients harbouring the gut microbiota from ob/ob mice have increased percentage body fat, increased energy consumption, as well as increased acetate and butyrate concentrations in the faecal samples [151]. This finding is echoed by a later study investigating the effect on adiposity by inoculating the microbiota from pairs of human twins, of whom one twin is obese, and the other is lean, in the germ-free mice. Upon the transplantation of microbiome, mice consume a low-fat (4%) and high-plant polysaccharides diet. However, mice become obese after human microbiome transplantation from the obese twin, whereas mice harbouring the human microbiome from the lean twin remains lean. When respective mice harbouring the lean and the obese microbiota are co-housed, both mice are resistant to obesity. The study further reports that the Bacteroidetes species in the gut microbiota from lean humans can resist the obese microbiota invasion [152]. These studies suggest that gut can be an origin of obesity.
High-fat diet induces endotoxemia with increased circulating lipopolysaccharides [153]. Lipopolysaccharide is the main component of gram-negative bacteria outer membrane, constituting a vast endotoxin reservoir in the gut. High-fat diet alters the ratio of gram-negative to gram-positive bacteria in the gut microbiome with an elevated composition of gram-negative bacteria [153,154]. Concomitantly, both dietary fat and intestinal dysbiosis reduce the integrity of the intestinal lumen [155,156]. The increased gut barrier permeability, also known as the leaky gut, results in the leakage of endotoxin [153,157]. The excess entry of gram-negative bacteria-derived lipopolysaccharides into the circulation results in endotoxemia and systemic inflammation [155,158,159,160].
Visceral adipose tissue is one of the target sites where lipopolysaccharides induce inflammation. 11-week lard diet increases the circulating lipopolysaccharide level and increases the expression levels of toll-like receptor 2 (TLR2) and toll-like receptor 4 (TLR4) when compared to mice receiving 11-week fish oil diet. Lipopolysaccharide binds to TLR4 on adipocyte, which in turn activates the Trif/MyD88/CCL2 signalling pathway [161,162,163]. TRIF and MyD88 are TLR adaptor molecules [164]. Chemokine CCL2 is a mediator of macrophage accumulation in white adipose tissue in obesity [165,166,167]. Interestingly, mice lacking TRIF and MyD88 are protected from lard diet-induced body weight gain and white adipose tissue inflammation. Trif-knockout and MyD88-knockout also prevent lard diet-induced CCL2 expression and inflammation in white adipose tissue as well as body weight gain. TLR4 recruits TRIF and MyD88, which promotes the expression of CCL2 in adipose tissue. The obesity-induced elevation of CCL2 level further recruits macrophage in white adipose tissue [168] (Figure 1).
The condition of the gut microbiome is shown to affect hippocampal plasticity. Young microbiome-free recipients harbouring the gut microbiota from the old mice promotes hippocampal neurogenesis and longevity. Conversely, microbiome-depleted recipient transplanted with faecal microbiota of high-fat diet-fed donor presents greater anxiety-like behaviour, which is accompanied by the increased expressions of lymphocyte and microglial marker in plasma and whole brain [169]. Further evidence shows that faecal microbiota transplant from 24-month-old donor mice impairs spatial learning and memory performance and novel object recognition in the young recipient [170]. These findings suggest that gut dysbiosis induces cognitive impairment. It is also possible that the gut has direct communication with the hippocampus [171]. Circuit study reveals a more direct connection between the gut-hippocampus axis. The medial nucleus tractus solitarius (mNTS) receives gut vagal sensory input, whereas the mNTS connects the dorsal CA3 region of the hippocampus through the medial septum [172]. Both the elimination of gastrointestinal vagal afferents and efferents by subdiaphragmatic vagotomy and selective gastrointestinal vagal deafferentation by injecting saporin-conjugated cholecystokinin into the nodose ganglia impair spatial memory and contextual episodic memory. The impaired hippocampal-dependent learning memory performances are accompanied by reduced hippocampal BDNF and doublecortin levels [172].
3. From Inflammation in Adipose Tissue to Impaired Hippocampus Plasticity in Obese and Diabetic Conditions
Obesity induces chronic inflammation in adipose tissues due to infiltration and activation of macrophages [173]. During extreme obesity, it is estimated that these macrophages can take up over 50% of all cells in the adipose tissue [174] and are polarized to the pro-inflammatory phenotype upon activation [175]. Several fat transplantation studies have demonstrated that the inflammatory response in adipose tissue could be linked to obesity and diabetes-impaired hippocampal plasticity and cognitive functions [55,176]. Leptin, a pro-inflammatory cytokine secreted from the adipose tissue, is elevated in obese and diabetic conditions [177,178,179]. Transplantation of epididymal fat from db/db (leptin receptor deficiency) donor to wild-type naïve mice activates microglia and macrophages in the hippocampus, and reduces dendritic spine density in the granule neurons, as well as impairs hippocampal long-term potentiation (LTP) [55]. These structural changes could be linked to spatial memory deficits in the hippocampal-dependent tasks [55]. Conversely, epididymal-lipectomy in db/db donor rescues spatial memory deficits, suppresses neuroinflammatory response, and restores hippocampal plasticity [55]. These findings have suggested that leptin resistance in adipose tissue could be a contributing factor to hippocampal plasticity deficits, and hence cognitive impairment.
The high-fat diet promotes the generation of reactive oxygen species (ROS) in the adipose tissue [180]. ROS can trigger nod-like receptor family, pyrin domain-containing 3 (NLRP3)-containing inflammasome to activate caspase-1 and secrete interleukin 1β (IL-1β) [181]. HFD induces obesity in Nlrp3 global knockout mice but prevents the development of adipose tissue inflammation and insulin resistance [182,183]. Increased circulating IL-1β level is associated with cognitive impairment in diabetes [184]. Visceral adipose tissue transplantation experiment in mice suggests that adipose tissue inflammation has a direct linkage to cognitive impairment with the associated neuroinflammatory response. Transplanting visceral adipose tissue from mice fed with 12-week HFS to wild-type recipient impairs spatial memory, activates microglial and increases hippocampal IL-1β levels [176]. On the contrary, fat transplantations from HFD-fed Nlrp3 −/− donors do not trigger neuroinflammatory response and memory deficits in recipients [176]. Other studies have shown that NLRP3-inflammasome/IL-1β signaling contributes to synaptic deficits in the hippocampus. The electrophysiological recording reveals that NLRP3 impairs LTP formation through IL-1 receptors [176]. Conversely, neutralizing IL-1 receptor rescues deficits in structural and synaptic plasticity in the hippocampus of the db/db mice [55]. In sum, these results have suggested that activation of NLRP3-inflammasome/IL-1β signalling could activate neuroinflammatory response and impair synaptic plasticity, leading to cognitive impairment in diet-induced obesity or diabetic condition (Figure 1).
Diet-induced obesity up-regulates the secretion of pro-inflammatory cytokines, whereas inflammatory response in adipose tissue further suppresses the secretion of adipocyte-derived anti-inflammatory cytokines, such as adiponectin [185]. Adiponectin is the most abundant adipokine in the bloodstream, which is secreted by mature adipocytes [186]. Adiponectin is an insulin-sensitizer by promoting glucose and fatty acid metabolism upon feeding [187]. In obese individuals, adiponectin secretion from white visceral adipose tissue decreases as adiposity increases [187]. Reduced insulin sensitivity, together with chronic inflammation, could progressively lead to systemic and central insulin resistance [24,188] (Figure 1). Adiponectin can cross the blood-brain barrier [130,189], suggesting its potential role in promoting insulin sensitivity in the brain. The functional role of adiponectin in the brain has recently been found using adiponectin knockout mouse models. Adiponectin activates AMPK to increase insulin sensitization in hippocampal and cortical neurons via suppressing Akt/GSK3β signaling [144]. Conversely, adiponectin deficiency disinhibits GSK3β-mediated cleavage of amyloid precursor protein and promotes plaque deposition [144], which in turn leads to neuroinflammation and hippocampal-dependent learning and memory deficits [144]. Adiponectin also mediates neuroinflammatory response through AdipoR1/NF-κB pathway [190]. Adiponectin deficiency induces microgliosis in the hippocampus and hypothalamus in an association with increased pro-inflammatory cytokine secretion [190]. Adiponectin also promotes hippocampal synaptic [132] and structural plasticity [131], which are abolished in adiponectin-deficient mice. Both obese and diabetic mice show reductions in adiponectin levels in the hippocampus [58,191]. These studies have collectively suggested that obesity and diabetes alter adiponectin secretion, which could consequently result in neurodegeneration and cognitive deficits.
Metabolic syndromes perturb adipose secretion of pro- and anti-inflammatory cytokines as well as adipose tissue inflammation. These perturbations not only affect the peripheral metabolism, but also trigger microglial activation in the hippocampus, and hence impair hippocampal plasticity. Studies have shown that withdrawal from the high-fat diet is an effective non-pharmacological intervention for improving learning and memory deficits [111,112]. Another study has also demonstrated that a feeding protocol with a 12-week high-fat diet, followed by an 8-week low-fat diet reduces microglial activation and increases spine density in the hippocampus [192]. The beneficial effect of dietary reversal is accompanied by reduced weight gain and fat masses [192]. Little is known about the interplay of microglial activation and adult neurogenesis in the obese and diabetic brain. However, it is shown that aberrant microglial activity may predominate the interplay by internalizing synaptic terminals upon chronic HFD while switching from HFD to a low-fat diet (LFD) attenuates synaptic internalization [192]. A recent study has reported that fractalkine receptor (CX3CR1) is involved in the microglial-mediated synaptic stripping in obese mice [193]. Obesity induces the expression of the phagocytic marker in hippocampal microglia [193], whereas Cx3cr1-haploinsufficiency counteracts spatial learning and memory deficits, microglial activation, and dendritic spine loss in the hippocampus [193]. CX3CR1 mediates microglial motility and activation [194]. Pharmacological blockade of microglial phagocytosis by annexin-V or suppressing microglial activity by minocycline prevents obesity-induced spine lost and cognitive impairment [193]. In sum, these findings have suggested the predominant role of microglia in inducing deficits in hippocampus structural plasticity in obesity (Figure 2).
4. Neuronal Mitochondria Are Involved in Obesity- or Diabetes-Induced Neuroinflammation and Hippocampal Impairment
Restoring or enhancing brain energetics has been proposed to be a potential therapeutic approach to halt neurodegenerative diseases of ageing [195]. Mitochondrial perturbation is observed in obese and diabetic conditions [196,197,198]. Independent of genetic factor, hyperglycemia contributes to the generation of reactive oxygen species (ROS) [199,200]. Excessive ROS production can induce mitochondrial DNA damage, lipid peroxidation, as well as oxidative phosphorylation (OXPHOS) [201]. These perturbations could increase the production of ROS and reduce oxidative capacity [202].
Diabetes-induced learning and memory deficits are associated with reduced mitochondrial density and ATP formation in neurons [203]. PGC-1α is a master regulator of mitochondrial biogenesis [204]. HFD induces insulin resistance and remarkably reduces expressions of transcriptional co-regulator of mitochondrial biogenesis (PGC-1α), mitochondrial transcription factor A (TFAM) and the mitochondrial OXPHOS complexes in the hippocampus [205]. Prominently, PGC-1α mediates the formation and maintenance of neuronal dendritic spines. siRNA-mediated PGC-1α silencing reduces dendritic spine density of granule neurons in the mouse hippocampal DG region. Consistently, PGC-1α silencing not only reduces dendritic spin density in primary hippocampal neurons [206], but also reduces mitochondrial density and ATP formation [206]. Conversely, activating BDNF/PGC-1α signaling cascade promotes synaptogenesis in hippocampal neurons [206], suggesting the role neuronal mitochondria on regulating synaptic plasticity through PGC-1α signaling.
Other studies have further revealed the alternative linkage between mitochondria-mediated dendritic spine integrity and microglial activity. Photo-ablation of mitochondria triggers dendritic spine clearance and dendritic retraction in primary hippocampal neurons, which activates caspase-3 in hippocampal dendrites [207]. Increased caspase-3 expression in the hippocampus is observed in diabetic rodent models [208,209]. Phospholipid scramblase and flippase are two families of enzymes that catalyze the translocations of phospholipids between the two monolayers of the cell membrane. Caspase-3 can, in turn, activate phospholipid scramblase and inhibit flippase [210,211], which exposes phosphatidylserine on the synaptic membrane. Microglia can recognize phosphatidylserine as an 'eat-me' signal and engulfing degenerating synapse [212]. Caspase-3 activation in the hippocampus of diabetic rodents may trigger a similar mechanism to recruit microglia for phagocytosis of damaged dendritic spines. Furthermore, autophagy could ameliorate the diabetes-induced metabolic crisis, apoptosis, and necrosis in neurons [213] (Figure 2).
In addition, insulin resistance could be linked to mitochondrial malfunction [214]. Adiponectin increases insulin sensitivity. Muscle-specific knockout of adiponectin receptor 1 (AdipoR1) results in systemic insulin resistance and hyperglycemia [215], resembling diabetic condition. Reduction in mitochondrial content and activity in the skeletal muscle could be linked to decreased exercise endurance in AdipoR1 knockout mice [215]. Mitochondrial activity and oxidative phosphorylation in skeletal muscles are found to be regulated by adiponectin/AdipoR1/AMPK/SIRT1/PGC-1α signalling pathway [215]. A proteomic study has revealed that AdipoR1 knockout impairs signalling pathways that are important for mitochondrial functioning, including as oxidative phosphorylation, TCA cycle, β-oxidation [216]. The expression of nuclear respiratory factor 1 (NRF1), a downstream target of PGC-1α and involves in mitochondrial biogenesis, OXPHOS, and ROS scavengers [217], is downregulated in both AdipoR1 −/− and HFD-fed mice, [216]. Adiponectin deficiency results in central insulin resistance [144], impairment in hippocampal plasticity [131,132], and cognitive function [132,144]. Adiponectin deficiency triggers Aβ oligomerization [144], whereas Aβ assault reduces mitochondrial membrane potential in primary hippocampal neurons which could be restored by activation of adiponectin signalling using receptor agonist, AdipoRon [218]. AdipoR1 knockout mice also display cognitive impairment [219]. These findings have provided potential linkage of how adiponectin signalling regulates mitochondrial function. The sophisticated mechanisms of how adipokines modulate neuronal mitochondria health and consequently affects neuronal functions warrant further investigation.
5. Excessive Palmitate Consumption from Diet Triggers a Direct Neuroinflammatory Response in the Hippocampus
Palmitate is the most abundant saturated fatty acid present in the circulation [220] and cerebrospinal fluid [221]. Increased brain uptake and accumulation of palmitate is reported in individuals with obesity and metabolic syndromes [222]. Moreover, palmitate is increased in the cerebrospinal fluid of overweight and obese humans [223]. Studies have suggested that palmitate impairs synaptic plasticity by elevated microglial activity. Palmitates suppress LTP formation in the Schaffer-collateral path [223]. Local infusion of palmitate in the cerebral ventricles impairs learning and memory performance in the object recognition task, object location task, step-down task, and the Barnes maze task [223]. In vitro study suggests that IRS-1 signalling is suppressed in the hippocampal neurons by the microglial-derived TNF-α [223], implicating palmitate-induced microglial activities may reduce insulin sensitivity in the hippocampal neurons. In another study, exosome fraction, which is isolated from palmitate-stimulated microglia in vitro, induces an immature dendritic spine phenotype in primary hippocampal neurons [224]. Lastly, high-fat diet also induces similar predominance of immature dendritic spines from CA1 neurons alongside with diminished levels of the scaffold protein Shank2 and impaired spatial memory performance [224]. These studies highlight the microglia-neuronal communication through exosomes, where palmitate-induced microglial inflammation may adversely influence on spine growth in the neighbouring neurons.
6. Obese and Diabetic Conditions Inhibit Hippocampal Feedback Control of the HPA Axis
An epidemiological study has reported that type 2 diabetic individuals have a higher prevalence of hypercortisolemia [225] while hypercorticosteronemia is also observed in diet-induced obese and genetically diabetic rodent models [226,227]. The increased activity in the hypothalamic-pituitary-adrenal axis (HPA) is a major cause of elevated cortisol or corticosterone secretion from the adrenal gland [228]. Lesion studies have illustrated that the hippocampus exerts efferent inhibitory control of the HPA axis. It is reported that total hippocampectomy elevates the expression of corticotropin-releasing hormone in the hypothalamic paraventricular nucleus (PVN) [229], whereas glucocorticoid secretion is markedly increased in rats bearing lesion in the ventral hippocampus [104]. Besides, recent circuit studies have revealed that the glutamatergic projections from ventral hippocampus synapses on the bed nucleus of striatum terminalis (BNST), whereby the BNST GABAergic projection elicits an inhibitory control over PVN [124,230]. It is reasonable to speculate that obese- or diabetes-induced hippocampal impairment may abolish the efferent inhibitory control over the HPA axis leading to hypercorticism. The presence of glucocorticoid receptors [231] and the ability that corticosterone can cross the blood-brain barrier [232] further implicate that the inhibitory action of hippocampus on HPA axis involves feedback inhibition. The feedback inhibition mechanism is supported by the fact that deletion of glucocorticoid receptors in the corticolimbic forebrain, including the hippocampal region, prolongs HPA axis activation [233,234].
Acute exposure of the high-fat diet for three days is sufficient to elevate corticosterone levels in rats and triggers neuroinflammatory responses with increased inflammasome-associated NLRP3 and CX3CR1 expressions in the hippocampus [102]. Acute HFD exposure leads to a higher vulnerability to a lipopolysaccharide-triggered inflammatory response with much higher hippocampal IL-1β and IL-6 expressions [102]. Independent of hypercorticosteronemia, systemic administration of a blood-brain barrier permeable glucocorticoid receptor antagonist: mifepristone [235] (also known as RU-486; 50 mg/kg s.c., two doses in three days) suppresses the expressions of neuroinflammatory markers in the hippocampus and attenuates lipopolysaccharide-induced neuroinflammation [102]. In addition to the pro-inflammatory effect of corticosterone, corticosterone impairs hippocampal plasticity, abrogates learning and memory performance, and reduces BDNF expression [106,108,236,237,238]. Conversely, mifepristone administration not only rescues memory deficits in an object recognition task but also restores LTP deficit in the Schaffer-collateral path in three-week-old juvenile rats exposed to the high-fat diet for 7 to 9 days [239]. Potentially, the high-fat diet-induced corticosterone surge impairs hippocampal function and plasticity, which in turn weakens the efferent inhibitory control over the HPA axis, where hypercorticosteronemia is observed in obesity and diabetes.
Conversely, hypercorticosteronemia-induced neuroinflammation and impaired hippocampal plasticity in obesity and diabetes can be intervened by normalizing the circulating corticosterone level or blocking the central action of corticosterone. Systemic administration of metyrapone reduces circulating corticosterone level by suppressing corticosterone synthesis [240,241]. In dependent of insulin sensitivity in the hippocampus, metyrapone administration in five-week-old db/db mice (100 mg/kg i.p., three weeks) reduces microgliosis and suppresses the expressions of IL-1β and TNFα in the hippocampus [242]. In vitro study has further illustrated that metyrapone administration reduces the pro-inflammatory M1 microglia population in the hippocampus of db/db mice as well as reduces microglial sensitivity against lipopolysaccharide assault in primary microglia culture [242]. These findings emphasize that the direct pro-inflammatory effect of corticosterone in the hippocampus regardless of a short-term high-fat diet exposure context or a genetically diabetic background. In db/db and streptozotocin-induced diabetic mice, adenectomy with concomitant corticosterone replacement restores cell proliferation and LTP formation in the hippocampus as well as improves learning and memory performance [44]. These restorations are linked to the reduction in circulating corticosterone levels and are independent of diabetes-induced hyperglycaemia [44]. In situ hybridization further reveals that adenectomy and corticosterone replacement restores BDNF-TrkB expressions in the hippocampal dentate region of db/db mice [243]. Moreover, suppression of corticosterone synthesis by systemic metyrapone administration in db/db mice rescues spatial memory deficits in concurrent with restored LTP formation and spine density of the granule cells in the hippocampus [227]. Systemic metyrapone administration fails to rescue the corticosterone-induced memory impairment, LTP deficits, and spine loss when corticosterone is locally infused in the hippocampus [227], suggesting the direct central action of corticosterone.
Corticosterone fails to induce spatial memory and synaptic impairments in db/db mice when glucocorticoid receptors are knocked down in the hippocampus [227], suggesting the potential role of glucocorticoid receptor signalling in the corticosterone action. Corticosterone impairs the hippocampal BDNF signalling pathway in db/db mice by two means. Firstly, the activation of glucocorticoid receptor transcriptionally suppresses the hippocampal BDNF expression. Concomitantly, the expressions of BDNF receptors are altered in db/db mice. While BDNF has a higher affinity for TrkB receptor, which promotes neural plasticity [244], it has a lower affinity for the P75 neurotrophin receptor (P75NTR) [245], which constrains plasticity [246]. TrkB expression is down-regulated, and P75NTR expression is up-regulated in the hippocampus of db/db mice, while pharmacological inhibition of corticosterone synthesis by metyrapone can re-activate the BDNF/TrkB cascade and bypass the P75NTR pathway. These studies demonstrate the complexity of the negative feedback mechanism of the hippocampus-HPA axis in response to obesity and diabetes-induced hypercorticosteronemia, in which the action of corticosterone on activating inflammasome-associated response and suppressing BDNF signaling induces neuroinflammation and plasticity deficits in the hippocampus (Figure 3).
7. High-Fats and Low-Carb Ketogenic Diet Elicits Neuroprotective Effects by Promoting Mitochondrial Dynamics and Reducing Oxidative Stress
There are misconceptions that high fat and dietary carbohydrate intake is directly linked to metabolic syndrome. Instead, high-fat diet elicits obesogenic effect when the caloric balance is upset by a high caloric intake versus a low energy expenditure, for example, physical inactivity. Counterintuitively, a high-fat, low-carbohydrate ketogenic diet (up to 90% fats in rodent studies) has been employed as a non-pharmacological diet intervention for controlling weight gain, glycemic index, and lipid content [247] and prevention for the recurrences of epilepsy [248].
Adopting a high-fat and very low-carbohydrates diet mimics the metabolic states of fasting and prolonged physical exercise, and elevates the levels of ketone bodies, known as physiologic ketosis [249]. When glucose reserve is depleted, lipids become the main source for ATP biosynthesis. Triacylglyceride is catalyzed into acetyl-CoA through β-oxidation, which then metabolized into ketone bodies, including acetone and β-hydroxybutyrate. β-hydroxybutyrate is the substrate for ATP biosynthesis. Ketosis in physiological state is different from diabetic ketoacidosis. Diabetic ketoacidosis, on the other hand, is a life-threatening complication of diabetes mainly due to the massive breakdown of lipids in adipose tissue induced by insulin deficit [250]. The high free fatty acid in the circulation accounts for approximately 80% of energy through β-oxidation, leading to the accumulation of ketone bodies (20–25 mM) at a level much higher than that during fasting or consuming a ketogenic diet (4–6 mM) [251,252,253,254,255,256].
Ketogenic diet elicits anti-inflammatory effect on adipose tissues under short-term consumption. Short-term exposure to a ketogenic diet (89.5% fats; 0.1% carbohydrates) for one week promotes ketogenesis with increased circulating β-hydroxybutyrate level in fed and fasting states [257]. Short term ketogenic exposure reduces the population of pro-inflammatory macrophages in the adipose tissue and down-regulates the Nlrp3 and Il1b expressions in the adipose-resident immune cells. In another study, it is also reported that four-week ketogenic diet (93.4% fat, 1.8% carbohydrate) down-regulates the expressions of inflammatory markers, such as Il6, Tnf, and Nlrp3, in the epidydimal white adipose tissue [258]. On the contrary, chronic exposure to a ketogenic diet (89.5% fats; 0.1% carbohydrates) for four months impose a detrimental effect on adipose tissue metabolism [257]. Chronic exposure promotes body weight gain and hyperglycemia [257]. Macrophage population is significantly increased in association with up-regulated inflammatory marker expressions, including Mcp1, Tnfa, and Il1b, in the epididymal fat tissues [257]. Still, the circulating β-hydroxybutyrate level remains high after four months of consuming a ketogenic diet. After that, an independent study attempts to harness the beneficial effect of ketogenesis by adopting a cyclic ketogenic diet paradigm. From 12-month-old onwards, naïve mice receive an alternating ketogenic diet (90% fats; 0% carbohydrates) and standard chow (13% fats; 77% carbohydrates) weekly until 36 months old [259]. Cyclic ketogenic diet elevates blood β-hydroxybutyrate levels but does not induce body weight gain. Aged mice have better memory recall in place avoidance test (at 22 to 24 months old) and object recognition task (at 28 to 30 months old) on a cyclic ketogenic diet than receiving standard chow. Also, the cyclic ketogenic diet reduces early mortality in naïve mice at 12 to 24 months old.
The dynamics of the NAD+/NADH ratio implies mitochondrial activity with increased mitochondrial fission increases the NAD+ levels. The two-week ketogenic diet (6:1 fats-to-carbohydrates and proteins ratio) elevates hippocampal NAD+/NADH ratio in 11 to 14-week-old rats [260]. In vitro study suggests that β-hydroxybutyrate may contribute to the increase in NAD+/NADH ratio [261]. Poly-ADP ribose polymerase-1 (PARP1) serves as a DNA damage sensor and participates in DNA repair [262]. While NAD+ is a substrate for PARP1, DNA damage triggers PARP1-induced NAD+ depletion [263]. The ketogenic diet, on the other hand, reduces hippocampal PARP-1 and 8-hydroxy-2′-deoxyguanosine levels, suggesting a potential reduction in DNA damage or enhanced DNA repair activity. SIRT-1 activity reduces cell death and inflammation [264,265] and promotes neuronal survival [266,267,268], while ketogenic diet promotes the activities of nuclear sirtuins as well as the hippocampal Sirt1 expression [260]. Altogether, the findings have suggested that the ketogenic diet could exert neuroprotective by promoting mitochondrial dynamics and reducing oxidative stress in neurons, which may serve as a dietary intervention to prevent cognitive decline.
Regardless, the effect of a ketogenic diet (84% fats; 0% carbohydrates) presents an adverse effect in the transgenic mice expressing a mutated mitochondrial DNA repair enzyme (mutUNG1) selectively in forebrain neurons [269]. The continuous ketogenic diet for two to four months reduces hippocampal size in mutUNG1 mice. Immunofluorescence shows a reduction in neuronal marker expression and an elevation of astroglia marker expression in the hippocampus upon a ketogenic diet in mutUNG mice. The increased SOD2/VDAC1 ratio as well the increased SIRT1 and FIS1 expressions in the hippocampus suggests ketogenic diet activates mitochondrial antioxidant defences and mitochondrial fission in mutUNG mice. Alterations in mitochondrial morphology, as well as the distribution of mitochondria within a neuron, are observed. For example, ketogenic diet induces swelling of mitochondria in mutUNG mice, whereas mitochondria are accumulated in the soma with reduced mitochondrial density in the presynaptic terminal of the pyramidal neurons in the hippocampal CA1. Lastly, electronic microscopy illustrates that ketogenic diet reduces the density of GluN2A and GluN2B subunits while increases the density of GABAA α1 subunits in the hippocampal CA1 of mutUNG1 mice. This study calls for rectification on whether a ketogenic diet is a proper way to augment neurodegeneration and cognitive impairments in association with severe mitochondrial dysfunction.
It is noteworthy that the peripheral metabolic, neuroprotective, and pro-cognitive effects of the ketogenic diet have called for substantial investigations. The ketogenic response is effective under low blood glucose and low insulin levels. Hyperglycemia and insulin resistance are hallmarks of diabetic patients, and these conditions are often shared in AD patients and older adults.
8. SIRT1/SIRT3 Axis Potentiates the Pro-Cognitive Effects of Caloric Restriction and Intermittent Fasting
Sirtuins (SIRT) are NAD+-dependent deacetylases [270]. SIRT1 is a cytosolic and nuclear sirtuin modulates transcription factors through histone deacetylation [264,271,272,273,274]. SIRT3 is a mitochondrial sirtuin regulating β-oxidation [275]. SIRT1/SIRT3 axis aims to promote energy storage intracellularly and to resist oxidative stress [276,277].
In vivo studies illustrate that SIRT1 is a mediator of caloric restriction-induced mitochondrial biogenesis in mice [278,279,280], while Sirt1 knockout abrogates the metabolic benefits of caloric restriction [273,281]. In the hippocampus, 23 weeks of HFD reduces the expressions of Ppargc1a, Ppp1cb, Reln and Sirt1 [282]. Increased DNA methylation and decreased DNA hydroxymethylation are observed in the promoter region of Sirt1, implicating a diet-induced epigenetic modulation of Sirt1 [282]. On the contrary, caloric restriction promotes learning and memory performance as well as hippocampal synaptic plasticity by upregulating signaling transducers involved in mitochondrial biogenesis [283], including CREB phosphorylation and increased expressions of SIRT1, PGC-1α, nitric oxide synthase, and phosphoenolpyruvate carboxykinase in the hippocampus [283]. The activation of a mitochondrial biogenesis signaling pathway is associated with better learning and memory performance in the novel object recognition task and enhanced LTP formation in the Schaffer-collateral path in a neuronal-specific CREB-dependent manner [283].
SIRT3 is one of the SIRT1 deacetylation targets. HFD-induced obesity and ageing obliterate SIRT1-mediated deacetylation of SIRT3, which reduces SIRT3 activity and stability in the mouse model [284]. Intermittent caloric restriction, as short as one week to one month, promotes the hippocampal SIRT3 expression [285]. Besides, the anxiolytic and pro-cognitive effects of intermittent caloric restriction are abolished when Sirt3 is selectively knockout in the hippocampal neurons. Intermittent caloric restriction further impairs LTP formation in the Schaffer-collateral path of the Sirt3-deficient mice. Significantly, intermittent fasting reduces seizure incidence, restores hippocampal-dependent spatial memory performance, as well as rescues LTP deficits in AppNL-G- F mice of AD.
SIRT3 can deacetylate LKB1, resulting in AMPK activation and mitochondrial biogenesis [286]. Four-week intermittent fasting activates AMPK/PGC-1a/mTORc/COX4/ND-1 signaling cascade, suggesting that intermittent fasting promotes mitochondrial biogenesis in the hippocampus. The increased mitochondrial DNA content further evidences this result in the hippocampus in db/db mice [287]. Essentially, intermittent fasting also exerts pro-cognitive and neurotrophic effects by restoring diabetes-induced hippocampal-dependent spatial memory deficits and the morphology of postsynaptic density of the neurons in the hippocampal CA1 region in addition to the activation of BDNF/pCREB/PSD-95 signaling cascade [287].
In sum, SIRT1/SIRT3 axis is one of the molecular targets of caloric restriction-induced pro-cognitive and neurotrophic effects, partly due to its functional role in regulating mitochondrial biogenesis.
9. Physical Exercise-Induced Mediators on Promoting Metabolic and Synaptic Function in the Brain
Adipose tissue secrets IL-1β that can trigger the neuroinflammatory response in obesity- and diabetes, it also secrets endocrine hormones such as leptin [137] and adiponectin [58,130] to mediate pro-cognitive effects of physical exercise. The decrease in adiponectin levels in diabetic and obese patients could be due to chronic inflammation of adipocytes [288,289]. Accumulating evidence has suggested the function role of adipose tissues on influencing cognitive function, metabolic function, and neural plasticity. Physical exercise can effectively attenuate metabolic syndromes in obesity and diabetes by improving lipid profile, adipose tissue inflammation, and insulin resistance [290,291,292]. Wheel running is also shown to ameliorate memory deficit and amyloid deposition in HFD-fed AD mouse model [293]. Notably, physical exercise is not only useful in combating a wide range of neurocognitive and neuropsychiatric symptoms in obese and diabetic rodents, including improving learning and memory deficits [294,295,296,297,298,299,300,301,302,303], reducing depression-like behaviours [90,304], and improving uncontrolled appetitive behaviours in hyperphagia suppression test [305,306,307]. Restored adult neurogenesis, improved synaptic plasticity, and reduced neuroinflammation may altogether contribute to cognitive improvements in obese and diabetic rodents [66,67,295,308,309,310,311,312]. Mitochondria perturbation could disrupt metabolism from cellular to system levels [313]. The physical exercise shows opposite effects to promote cognitive function by promoting mitochondrial functions and reduce neuronal apoptosis in the hippocampus of mice fed with HFD [299].
Emerging data have found that metabolic factors secreted by peripheral organs are not only involved in regulating metabolism but also have a direct effect on neurodegeneration in association with metabolic disorders. Exerkines, including apelin secreted from the adipose tissues, β-hydroxybutyrate secreted by the liver, Fibronectin type III domain-containing protein 5 (FNDC5) secreted from the muscles, are involved in bridging the communication between the peripheral organs and the hippocampus. Functions of these factors on modulating hippocampal plasticity, neuroinflammation, and metabolic function in the brain are discussed below (Figure 4).
9.1. Apelin
Apelin is initially known as an adipokine [314] which is secreted by adipocytes. Hyperinsulinemia induces high circulating apelin levels in obesity [314]. Later studies have suggested apelin as a myokine because muscle contraction in young mice induces apelin secretion from the muscles [315]. Ageing suppresses apelin secretion from skeletal muscle while delivering exogenous apelin can rejuvenate the skeletal muscle strength in aged mice and apelin-deficient mice by promoting mitochondrial activity [315]. Overexpression of apelin also prevents HFD-induced obesity by promoting mitochondrial biogenesis and increasing energy expenditure in skeletal muscle [316]. Brain SIRT1, a mediator of neuroprotection and metabolic homeostasis [317], can be activated by apelin [318] and physical exercise [319,320]. Apelin receptor is abundantly expressed in rodent brains, including the hippocampus, hypothalamus, substantia nigra, entorhinal cortex [321]. Apelin suppresses inflammation-related NF-κB signaling by activating SIRT1 in the hippocampus [318]. Furthermore, apelin modulates microglia activation by attenuating the polarization of microglia to M1 pro-inflammatory phenotype under chronic stress condition [322]. Other than suppressing inflammatory responses, treatment with recombinant apelin-13 elicits anxiolytic [318] and antidepressant effects [322], and reduces the inflammatory response. Genetic knockout of Nlrp3, downstream target of NF-κB, obliterate chronic stress-induced inflammatory response and depressive behaviors [323]. Activation of SIRT1 is required to promote mitochondrial biogenesis as well as lipid and glucose metabolism [324,325]. In vitro study reveals mitochondria could mediate the neuroprotective effect of apelin-13. Apelin-13 preserves mitochondrial integrity and reduces the production of reactive oxygen species [ROS], neuronal apoptosis and NMDA-induced excitotoxicity in primary cortical neurons [326]. However, it is still largely unknown whether the antidepressant and anxiolytic effects of apelin are mediated by modulating microglia activity in the brain.
9.2. Irisin
Irisin or fibronectin type III domain-containing protein 5 (FNDC5) is a myokine secreted by muscle cells. It can stimulate adipocyte browning and thermogenesis [327,328]. Diabetic patients show significant reductions in circulating levels of irisin and expression levels of Fndc5 in adipose tissue. Conversely, the expression level of Fndc5 in skeletal muscle is upregulated in diabetic conditions. In obese mice, overexpression of Fndc5 or subcutaneous administration with recombinant irisin reduces hyperglycemia, hyperlipidemia, and improves insulin resistance [329]. Voluntary wheel running promotes Fndc5/Pgc1α/Erra expressions in both the quadriceps and hippocampus and upregulates Zif268, cFos, and Arc expressions in the hippocampus, implicating an exercise-induced neuronal activation [330]. Intrahippocampal infusion of irisin into the dentate region promotes spatial and avoidance learning in naïve Wistar rats [331], possibly by promoting LTP formation [332]. Congruently, over-expression of irisin in the brain can restore hippocampal BDNF levels in concurrent with HFD/streptozotocin-induced diabetic condition in SD rats [333]. Irisin can cross the BBB [330]. However, peripheral neutralization of FNDC5 abolishes the pro-cognitive and neurotrophic effects of swimming exercise [334], suggesting peripheral irisin could mediate muscle-brain crosstalk. BDNF is a downstream target of irisin, while glucose metabolism is regulated by irisin in the brain [333]. Peripheral overexpression of Fndc5 also promotes hippocampal BDNF levels [330]. It is noted that FNDC5 can also be synthesized in the brain. A recent study has suggested that exercise-induced lactate secretion from the muscle or exogenous lactate delivery promotes learning and memory through activating hippocampal endothelial monocarboxylate transporters/SIRT1/PGC-1α/FNDC5/BDNF signaling pathway [335]. Another study investigating the role of irisin on mood regulation demonstrates that irisin is a mediator of exercise-induced antidepressant and neurotrophic effect, while the irisin mechanism of antidepressant action requires more in-depth investigations [336]. These studies have highlighted the pro-cognitive [335,336] and neurotrophic effect [336,337] of myokines on the hippocampus.
9.3. Lactate
Lactate is another myokine that is released from muscle in response to physical exercise. Lactate can cross the blood-brain barrier via endothelial monocarboxylate transporters [338,339]. The lactate action could be elicited by activating the lactate receptor HCAR1 in the brain. HCAR1 is present in the fibroblasts and ependymal cells in the brain [340]. HCAR1 mediates the lactate-induced neuronal excitability in primary cortical neurons [341] as well as high-intensity exercise-induced or lactate-induced angiogenesis in the brain [342]. Interestingly, systemic lactate administration partially mimics the wheel running effect on modulating the gene expression that regulates mitochondrial biogenesis in the liver and the whole brain. Lactate administration mimics the effect of exercise by upregulating the gene expressions of PGC-1α but downregulating the expressions of PGC-1β and NRF-1 in the liver. Besides, both lactate administration and exercise increase the PGC-1 related co-activator (PRC)/vascular endothelial growth factor A (VEGF-A) expression in the brain. Of note, exercise increases the mitochondrial DNA copy number in the brain and reduces TNFα expression but not after lactate administration [339]. Other pro-cognitive effects, including anti-depressant effects, are illustrated upon single-dose of lactate administration in corticosterone-induced stressed mice as well as after chronic lactate administration in naïve mice [343].
An earlier study has demonstrated the involvement of lactate in promoting neurogenesis and vascularization in the postnatal mouse brain [344]. Extracellular electrophysiological recordings have further revealed that lactate promotes synaptic plasticity at the Schaffer-collateral path [345]. In the CA3 pyramidal neurons, lactate acts on the postsynaptic lactate receptor and may facilitate AMPAR insertion in a PI3K-dependent manner, which is evidenced by increased intrinsic excitability of CA3 pyramidal neurons [345]. Potentially, lactate promotes NMDAR-mediated calcium ion influx through PI3K/PKC phosphorylation, as illustrated by an increased EPSP-spike coupling [345].
Lactate was known as a by-product of anaerobic respiration, but it is also a source of energy, a gluconeogenic precursor, and a signaling molecule [346]. Since then, the concept of lactate shuttle has been raised with two highlights. First, lactate can be exported and consumed by the neighboring cells through lactate receptors [paracrine route] or by a distal organ through circulation [endocrine route] [346]. Second, the role of lactates shifts from glycolytic to oxidative in the recipient tissues and organs [346]. The exercise-induced lactate/MCT/SIRT1/PGC-1α/FNDC5/BDNF signaling transduction has exemplified the endocrine perspective of lactate shuttle [335]. Also, several reviews have emphasized the lactate shuttle involving in synaptic plasticity and astroglial-neuronal metabolism [347,348,349,350,351]. Lactate enters the presynaptic neurons through monocarboxylate transport 2 (MCT2) [352]. In the presynaptic neurons, lactate plays an oxidative role in fueling synaptic plasticity [353]. During synaptic activation, glutamate released in the synaptic cleft is taken up by astrocytes to maintain glutamate homeostasis [354]. Simultaneously, astrocytes continue to take up glucose from the brain circulation [355], while glucose is converted into lactate by glycolysis and lactic acid fermentation [356]. Astrocytic lactate is then shuttled to the neighboring presynaptic neurons through astrocytic monocarboxylate transporters 1 and 4 (MCT1/4) and neuronal MCT2 [357]. This idea has been recently exemplified by adenovirus-mediated conditional knockout of neuronal MCT2 and astrocytic MCT4 in a rodent study. Conditional knockout of neuronal MCT2 and astrocytic MCT4 impair learning and memory performances in the hippocampus-dependent tasks [358]. Importantly, lactate infusion in the cerebral ventricles restores spatial learning in MCT4 knockout mice, suggesting that lactate shuttling by the astrocytes is pivotal to the direct effect of lactate, which contributes to hippocampal-dependent learning [358]. Notably, neuronal MCT2-knockout, but not astrocytic MCT4-knockout, reduces the immature neuron population in the hippocampal dentate gyrus [358], suggesting MCT2 is involved in regulating adult neurogenesis in the hippocampus. All in all, these results have supported the lactate shuttle hypothesis and how astroglial-neuron shuttle is involved in hippocampal-dependent learning and memory.
9.4. β-Hydroxybutyrate
β-Hydroxybutyrate is a hepatokine, which is synthesized in the liver from fat circulating in the bloodstream during fasting and exercise [359]. During diabetic ketoacidosis, increased β-oxidation and acidosis reduces mitochondrial redox state in favour of β-hydroxybutyrate biosynthesis in the liver [360]. Conversely, running exercise elevates the circulating level of β-hydroxybutyrate [361]. β-hydroxybutyrate seems to elicit both anti-inflammatory and neurotrophic effects. β-hydroxybutyrate is an endogenic NLRP3 inflammasome inhibitor [362,363], while subcutaneous administration can reduce IL-1β and TNF-α levels in the hippocampus after acute immobilization and chronic unpredictable stress [364]. Intragastric administration of β-hydroxybutyrate improves spatial learning and memory in naïve rats [365] and AD mice [366]. Another study further demonstrates that wheel running exercise increases hippocampal β-hydroxybutyrate levels in mice [367]. Intraventricular infusion of β-hydroxybutyrate mimics the exercise effect by upregulating Bdnf expression in the hippocampus, possibly through suppressing HDAC2/3 [367]. Glutamatergic signalling in the Schaffer-collateral path is enhanced upon acute β-hydroxybutyrate incubation [367]. Moreover, oral administration of β-hydroxybutyrate to rats for five days improves their spatial learning and memory and enhances their endurance in a treadmill test [368]. Besides, in vitro study further illustrates that β-hydroxybutyrate stimulates mitochondrial respiration and increases ATP formation in cultured cortical neurons [361]. The increased mitochondrial activity may further activate NF-κB/BDNF pathway to elicit neurogenic effect [361].
10. Conclusions
Emerging evidence has suggested that obesity and diabetic condition could increase the risk for neurodegeneration, and hence cognitive impairments. This review has summarized the experimental evidence demonstrating the potential influences of chronic inflammation in adipose tissue on inducing neuroinflammation and impairing neural plasticity in the brain under diabetic and obese condition. The chronic, low-grade inflammatory response in adipose tissue adversely suppresses the synthesis and secretion of anti-inflammatory adipokines, such as adiponectin. Elevated IL-1β level triggers an inflammatory response, whereas suppressed adiponectin secretion triggers insulin resistance. Aberrant peripheral secretion of adipokines in obesity and diabetes can trigger neuroinflammatory response, which in turn impairs synaptic plasticity and cognitive functioning. Mitochondria play a crucial role in maintaining neuroinflammatory response and neural plasticity in the brain. Obesity and diabetes impair mitochondrial function, that could also contribute to neuropathology, including spine loss, microglial activation, AD pathology in the brain. Physical exercise improves metabolic and brain functions. Factors including apelin, irisin, lactate, and β-hydroxybutyrate secreted from the adipose tissue, skeletal muscles, and liver are increased in response to physical exercise. These metabolic factors promote not only metabolic functions in the peripheral organs, but also maintain mitochondrial homeostasis and neural plasticity in the brain.
In sum, convergent evidence has illustrated that obesity or diabetes triggers neuroinflammation, insulin resistance, the mitochondrial perturbation in the brain, which could impair hippocampus neuroplasticity and hence cognitive function. These findings have highlighted that neurodegeneration could be accelerated by a chronic metabolic disorder, whereas targeting some key metabolic factors that respond to physical exercise could pave the way for therapeutics and augment deficits in both metabolism and neurodegeneration.
Author Contributions
T.H.-y.L. wrote the manuscript and designed graphics. S.-y.Y. revised and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The work was supported by funding awarded to SY YAU (Hong Kong Research Grant Council, Early career scheme: 25100217; General Research Fund: 15100018 and National Science Foundation of China, Young investigator scheme: 81801346).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kant, A.K. Dietary patterns and health outcomes. J. Am. Diet. Assoc. 2004, 104, 615–635. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.G.; Zimmet, P.; Shaw, J.; Group IDFETFC. The metabolic syndrome—A new worldwide definition. Lancet 2005, 366, 1059–1062. [Google Scholar] [CrossRef]
- Eckel, R.H.; Alberti, K.G.; Grundy, S.M.; Zimmet, P.Z. The metabolic syndrome. Lancet 2010, 375, 181–183. [Google Scholar] [CrossRef]
- Christ, A.; Lauterbach, M.; Latz, E. Western Diet and the Immune System: An Inflammatory Connection. Immunity 2019, 51, 794–811. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D. Dietary and policy priorities to reduce the global crises of obesity and diabetes. Nat. Food 2020, 1, 38–50. [Google Scholar] [CrossRef]
- Francis, H.M.; Stevenson, R.J. Higher reported saturated fat and refined sugar intake is associated with reduced hippocampal-dependent memory and sensitivity to interoceptive signals. Behav. Neurosci. 2011, 125, 943–955. [Google Scholar] [CrossRef]
- Gibson, E.L.; Barr, S.; Jeanes, Y.M. Habitual fat intake predicts memory function in younger women. Front. Hum. Neurosci. 2013, 7, 838. [Google Scholar] [CrossRef]
- Baym, C.L.; Khan, N.A.; Monti, J.M.; Raine, L.B.; Drollette, E.S.; Moore, R.D.; Scudder, M.R.; Kramer, A.F.; Hillman, C.H.; Cohen, N.J. Dietary lipids are differentially associated with hippocampal-dependent relational memory in prepubescent children. Am. J. Clin. Nutr. 2014, 99, 1026–1032. [Google Scholar] [CrossRef]
- Gardener, S.L.; Rainey-Smith, S.R.; Barnes, M.B.; Sohrabi, H.R.; Weinborn, M.; Lim, Y.Y.; Harrington, K.; Taddei, K.; Gu, Y.; Rembach, A.; et al. Dietary patterns and cognitive decline in an Australian study of ageing. Mol. Psychiatry 2015, 20, 860–866. [Google Scholar] [CrossRef]
- Brannigan, M.; Stevenson, R.J.; Francis, H. Thirst interoception and its relationship to a Western-style diet. Physiol. Behav. 2015, 139, 423–429. [Google Scholar] [CrossRef]
- Granic, A.; Davies, K.; Adamson, A.; Kirkwood, T.; Hill, T.R.; Siervo, M.; Mathers, J.C.; Jagger, C. Dietary Patterns High in Red Meat, Potato, Gravy, and Butter Are Associated with Poor Cognitive Functioning but Not with Rate of Cognitive Decline in Very Old Adults. J. Nutr. 2016, 146, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Attuquayefio, T.; Stevenson, R.J.; Boakes, R.A.; Oaten, M.J.; Yeomans, M.R.; Mahmut, M.; Francis, H.M. A high-fat high-sugar diet predicts poorer hippocampal-related memory and a reduced ability to suppress wanting under satiety. J. Exp. Psychol. Anim. Learn. Cogn. 2016, 42, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Attuquayefio, T.; Stevenson, R.J.; Oaten, M.J.; Francis, H.M. A four-day Western-style dietary intervention causes reductions in hippocampal-dependent learning and memory and interoceptive sensitivity. PLoS ONE 2017, 12, e0172645. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, R.J.; Francis, H.M.; Attuquayefio, T.; Gupta, D.; Yeomans, M.R.; Oaten, M.J.; Davidson, T. Hippocampal-dependent appetitive control is impaired by experimental exposure to a Western-style diet. R. Soc. Open Sci. 2020, 7, 191338. [Google Scholar] [CrossRef] [PubMed]
- Neth, B.J.; Craft, S. Insulin Resistance and Alzheimer's Disease: Bioenergetic Linkages. Front. Aging Neurosci. 2017, 9, 345. [Google Scholar] [CrossRef]
- Ferreira, L.S.S.; Fernandes, C.S.; Vieira, M.N.N.; De Felice, F.G. Insulin Resistance in Alzheimer's Disease. Front Neurosci. 2018, 12, 830. [Google Scholar] [CrossRef]
- Arnold, S.E.; Arvanitakis, Z.; Macauley-Rambach, S.L.; Koenig, A.M.; Wang, H.Y.; Ahima, R.S.; Craft, S.; Gandy, S.; Buettner, C.; Stoeckel, L.E.; et al. Brain insulin resistance in type 2 diabetes and Alzheimer disease: Concepts and conundrums. Nat. Rev. Neurol. 2018, 14, 168–181. [Google Scholar] [CrossRef]
- Hamer, J.A.; Testani, D.; Mansur, R.B.; Lee, Y.; Subramaniapillai, M.; McIntyre, R.S. Brain insulin resistance: A treatment target for cognitive impairment and anhedonia in depression. Exp. Neurol. 2019, 315, 1–8. [Google Scholar] [CrossRef]
- Wakabayashi, T.; Yamaguchi, K.; Matsui, K.; Sano, T.; Kubota, T.; Hashimoto, T.; Mano, A.; Yamada, K.; Matsuo, Y.; Kubota, N. Differential effects of diet- and genetically-induced brain insulin resistance on amyloid pathology in a mouse model of Alzheimer's disease. Mol. Neurodegener. 2019, 14, 15. [Google Scholar] [CrossRef]
- Vandal, M.; White, P.J.; Chevrier, G.; Tremblay, C.; St-Amour, I.; Planel, E.; Marette, A.; Calon, F. Age-dependent impairment of glucose tolerance in the 3xTg-AD mouse model of Alzheimer's disease. FASEB J. 2015, 29, 4273–4284. [Google Scholar] [CrossRef]
- Macklin, L.; Griffith, C.M.; Cai, Y.; Rose, G.M.; Yan, X.X.; Patrylo, P.R. Glucose tolerance and insulin sensitivity are impaired in APP/PS1 transgenic mice prior to amyloid plaque pathogenesis and cognitive decline. Exp. Gerontol. 2017, 88, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Griffith, C.M.; Macklin, L.N.; Cai, Y.; Sharp, A.A.; Yan, X.X.; Reagan, L.P.; Strader, A.D.; Rose, G.M.; Patrylo, P.R. Impaired Glucose Tolerance and Reduced Plasma Insulin Precede Decreased AKT Phosphorylation and GLUT3 Translocation in the Hippocampus of Old 3xTg-AD Mice. J. Alzheimers Dis. 2019, 68, 809–837. [Google Scholar] [CrossRef] [PubMed]
- Velazquez, R.; Tran, A.; Ishimwe, E.; Denner, L.; Dave, N.; Oddo, S.; Dineley, K.T. Central insulin dysregulation and energy dyshomeostasis in two mouse models of Alzheimer's disease. Neurobiol. Aging 2017, 58, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kothari, V.; Luo, Y.; Tornabene, T.; O'Neill, A.M.; Greene, M.W.; Geetha, T.; Babu, J.R. High fat diet induces brain insulin resistance and cognitive impairment in mice. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 499–508. [Google Scholar] [CrossRef]
- Selles, M.C.; Fortuna, J.T.S.; Zappa-Villar, M.F.; de Faria, Y.P.R.; Souza, A.S.; Suemoto, C.K.; Leite, R.E.P.; Rodriguez, R.D.; Grinberg, L.T.; Reggiani, P.C.; et al. Adenovirus-Mediated Transduction of Insulin-Like Growth Factor 1 Protects Hippocampal Neurons from the Toxicity of Abeta Oligomers and Prevents Memory Loss in an Alzheimer Mouse Model. Mol. Neurobiol. 2020, 57, 1473–1483. [Google Scholar] [CrossRef]
- Rhea, E.M.; Raber, J.; Banks, W.A. ApoE and cerebral insulin: Trafficking, receptors, and resistance. Neurobiol. Dis. 2020, 137, 104755. [Google Scholar] [CrossRef]
- Holscher, C. Brain insulin resistance: Role in neurodegenerative disease and potential for targeting. Expert Opin. Investig. Drugs. 2020, 29, 333–348. [Google Scholar] [CrossRef]
- Horvath, A.; Salman, Z.; Quinlan, P.; Wallin, A.; Svensson, J. Patients with Alzheimer's Disease Have Increased Levels of Insulin-like Growth Factor-I in Serum but not in Cerebrospinal Fluid. J. Alzheimers Dis. 2020, 75, 289–298. [Google Scholar] [CrossRef]
- Caberlotto, L.; Nguyen, T.P.; Lauria, M.; Priami, C.; Rimondini, R.; Maioli, S.; Cedazo-Minguez, A.; Sita, G.; Morroni, F.; Corsi, M.; et al. Cross-disease analysis of Alzheimer's disease and type-2 Diabetes highlights the role of autophagy in the pathophysiology of two highly comorbid diseases. Sci. Rep. 2019, 9, 3965. [Google Scholar] [CrossRef]
- Desai, G.S.; Zheng, C.; Geetha, T.; Mathews, S.T.; White, B.D.; Huggins, K.W.; Zizza, C.A.; Broderick, T.L.; Babu, J.R. The pancreas-brain axis: Insight into disrupted mechanisms associating type 2 diabetes and Alzheimer's disease. J. Alzheimers Dis. 2014, 42, 347–356. [Google Scholar] [CrossRef]
- De la Monte, S.M.; Wands, J.R. Alzheimer's disease is type 3 diabetes-evidence reviewed. J. Diabetes Sci. Technol. 2008, 2, 1101–1113. [Google Scholar] [CrossRef] [PubMed]
- Arnoldussen, I.A.; Kiliaan, A.J.; Gustafson, D.R. Obesity and dementia: Adipokines interact with the brain. Eur. Neuropsychopharmacol. 2014, 24, 1982–1999. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Cheng, K.K.; Hoo, R.L.; Siu, P.M.; Yau, S.Y. The Novel Perspectives of Adipokines on Brain Health. Int. J. Mol. Sci. 2019, 20, 5638. [Google Scholar] [CrossRef] [PubMed]
- Danat, I.M.; Clifford, A.; Partridge, M.; Zhou, W.; Bakre, A.T.; Chen, A.; McFeeters, D.; Smith, T.; Wan, Y.; Copeland, J.; et al. Impacts of Overweight and Obesity in Older Age on the Risk of Dementia: A Systematic Literature Review and a Meta-Analysis. J. Alzheimers Dis. 2019, 70, S87–S99. [Google Scholar] [CrossRef]
- Bowman, K.; Thambisetty, M.; Kuchel, G.A.; Ferrucci, L.; Melzer, D. Obesity and Longer Term Risks of Dementia in 65–74 Year Olds. Age Ageing 2019, 48, 367–373. [Google Scholar] [CrossRef]
- Lee, J.Y.; Han, K.; Han, E.; Kim, G.; Cho, H.; Kim, K.J.; Lee, B.W.; Kang, E.S.; Cha, B.; Brayne, C.; et al. Risk of Incident Dementia According to Metabolic Health and Obesity Status in Late Life: A Population-Based Cohort Study. J. Clin. Endocrinol. Metab. 2019, 104, 2942–2952. [Google Scholar] [CrossRef]
- Lee, A.T.C.; Richards, M.; Chan, W.C.; Chiu, H.F.K.; Lee, R.S.Y.; Lam, L.C.W. Higher dementia incidence in older adults with type 2 diabetes and large reduction in HbA1c. Age Ageing 2019, 48, 838–844. [Google Scholar] [CrossRef]
- Alsharif, A.A.; Wei, L.; Ma, T.; Man, K.K.C.; Lau, W.C.Y.; Brauer, R.; Almetwazi, M.; Howard, R.; Wong, I.C.K. Prevalence and Incidence of Dementia in People with Diabetes Mellitus. J. Alzheimers Dis. 2020, 75, 607–615. [Google Scholar] [CrossRef]
- Park, S.Y.; Cho, Y.R.; Kim, H.J.; Higashimori, T.; Danton, C.; Lee, M.K.; Dey, A.; Rothermel, B.; Kim, Y.; Kalinowski, A.; et al. Unraveling the temporal pattern of diet-induced insulin resistance in individual organs and cardiac dysfunction in C57BL/6 mice. Diabetes 2005, 54, 3530–3540. [Google Scholar] [CrossRef]
- Koranyi, L.; James, D.; Mueckler, M.; Permutt, M.A. Glucose transporter levels in spontaneously obese (db/db) insulin-resistant mice. J. Clin. Investig. 1990, 85, 962–967. [Google Scholar] [CrossRef]
- Tomita, T.; Doull, V.; Pollock, H.G.; Krizsan, D. Pancreatic islets of obese hyperglycemic mice (ob/ob). Pancreas 1992, 7, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Beddow, S.A.; Samuel, V.T. Fasting hyperglycemia in the Goto-Kakizaki rat is dependent on corticosterone: A confounding variable in rodent models of type 2 diabetes. Dis. Models Mech. 2012, 5, 681–685. [Google Scholar] [CrossRef] [PubMed]
- Boitard, C.; Etchamendy, N.; Sauvant, J.; Aubert, A.; Tronel, S.; Marighetto, A.; Laye, S.; Ferreira, G. Juvenile, but not adult exposure to high-fat diet impairs relational memory and hippocampal neurogenesis in mice. Hippocampus 2012, 22, 2095–2100. [Google Scholar] [CrossRef] [PubMed]
- Stranahan, A.M.; Arumugam, T.V.; Cutler, R.G.; Lee, K.; Egan, J.M.; Mattson, M.P. Diabetes impairs hippocampal function through glucocorticoid-mediated effects on new and mature neurons. Nat. Neurosci. 2008, 11, 309–317. [Google Scholar] [CrossRef]
- Sharma, A.N.; Elased, K.M.; Garrett, T.L.; Lucot, J.B. Neurobehavioral deficits in db/db diabetic mice. Physiol. Behav. 2010, 101, 381–388. [Google Scholar] [CrossRef]
- Leuner, B.; Gould, E. Structural plasticity and hippocampal function. Annu. Rev. Psychol. 2010, 61, 111–140. [Google Scholar] [CrossRef]
- Moser, M.B.; Moser, E.I. Functional differentiation in the hippocampus. Hippocampus 1998, 8, 608–619. [Google Scholar] [CrossRef]
- Smith, M.A. Hippocampal vulnerability to stress and aging: Possible role of neurotrophic factors. Behav. Brain Res. 1996, 78, 25–36. [Google Scholar] [CrossRef]
- Miller, D.B.; O'Callaghan, J.P. Aging, stress and the hippocampus. Ageing Res. Rev. 2005, 4, 123–140. [Google Scholar] [CrossRef]
- Bettio, L.E.B.; Rajendran, L.; Gil-Mohapel, J. The effects of aging in the hippocampus and cognitive decline. Neurosci. Biobehav. Rev. 2017, 79, 66–86. [Google Scholar] [CrossRef]
- Ho, N.; Sommers, M.S.; Lucki, I. Effects of diabetes on hippocampal neurogenesis: Links to cognition and depression. Neurosci. Biobehav. Rev. 2013, 37, 1346–1362. [Google Scholar] [CrossRef] [PubMed]
- Cherbuin, N.; Sargent-Cox, K.; Fraser, M.; Sachdev, P.; Anstey, K.J. Being overweight is associated with hippocampal atrophy: The PATH Through Life Study. Int. J. Obes. 2015, 39, 1509–1514. [Google Scholar] [CrossRef] [PubMed]
- Robison, L.S.; Albert, N.M.; Camargo, L.A.; Anderson, B.M.; Salinero, A.E.; Riccio, D.A.; Abi-Ghanem, C.; Gannon, O.J.; Zuloaga, K.L. High-Fat Diet-Induced Obesity Causes Sex-Specific Deficits in Adult Hippocampal Neurogenesis in Mice. eNeuro 2020, 7, ENEURO.0391-19.2019. [Google Scholar] [CrossRef]
- Ramos-Rodriguez, J.J.; Molina-Gil, S.; Ortiz-Barajas, O.; Jimenez-Palomares, M.; Perdomo, G.; Cozar-Castellano, I.; Lechuga-Sancho, A.M.; Garcia-Alloza, M. Central proliferation and neurogenesis is impaired in type 2 diabetes and prediabetes animal models. PLoS ONE 2014, 9, e89229. [Google Scholar] [CrossRef] [PubMed]
- Erion, J.R.; Wosiski-Kuhn, M.; Dey, A.; Hao, S.; Davis, C.L.; Pollock, N.K.; Stranahan, A.M. Obesity elicits interleukin 1-mediated deficits in hippocampal synaptic plasticity. J. Neurosci. 2014, 34, 2618–2631. [Google Scholar] [CrossRef] [PubMed]
- Bracke, A.; Domanska, G.; Bracke, K.; Harzsch, S.; van den Brandt, J.; Broker, B.; Bohlen, V.; Halbach, O. Obesity Impairs Mobility and Adult Hippocampal Neurogenesis. J. Exp. Neurosci. 2019, 13, 1179069519883580. [Google Scholar] [CrossRef]
- Porter, W.D.; Flatt, P.R.; Holscher, C.; Gault, V.A. Liraglutide improves hippocampal synaptic plasticity associated with increased expression of Mash1 in ob/ob mice. Int. J. Obes. 2013, 37, 678–684. [Google Scholar] [CrossRef]
- Yau, S.Y.; Lee, T.H.; Li, A.; Xu, A.; So, K.F. Adiponectin Mediates Running-Restored Hippocampal Neurogenesis in Streptozotocin-Induced Type 1 Diabetes in Mice. Front. Neurosci. 2018, 12, 679. [Google Scholar] [CrossRef]
- Yan, S.; Du, F.; Wu, L.; Zhang, Z.; Zhong, C.; Yu, Q.; Wang, Y.; Lue, L.; Walker, D.G.; Douglas, J.T.; et al. F1F0 ATP Synthase-Cyclophilin D Interaction Contributes to Diabetes-Induced Synaptic Dysfunction and Cognitive Decline. Diabetes 2016, 65, 3482–3494. [Google Scholar] [CrossRef]
- Li, X.H.; Xin, X.; Wang, Y.; Wu, J.Z.; Jin, Z.D.; Ma, L.N.; Nie, C.; Xiao, X.; Hu, Y.; Jin, M. Pentamethylquercetin protects against diabetes-related cognitive deficits in diabetic Goto-Kakizaki rats. J. Alzheimers Dis. 2013, 34, 755–767. [Google Scholar] [CrossRef]
- Yin, H.; Wang, W.; Yu, W.; Li, J.; Feng, N.; Wang, L.; Wang, X. Changes in Synaptic Plasticity and Glutamate Receptors in Type 2 Diabetic KK-Ay Mice. J. Alzheimers Dis. 2017, 57, 1207–1220. [Google Scholar] [CrossRef] [PubMed]
- Nicola, Z.; Fabel, K.; Kempermann, G. Development of the adult neurogenic niche in the hippocampus of mice. Front. Neuroanat. 2015, 9, 53. [Google Scholar] [CrossRef] [PubMed]
- Beauquis, J.; Saravia, F.; Coulaud, J.; Roig, P.; Dardenne, M.; Homo-Delarche, F.; De Nicola, A. Prominently decreased hippocampal neurogenesis in a spontaneous model of type 1 diabetes, the nonobese diabetic mouse. Exp. Neurol. 2008, 210, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Lindqvist, A.; Mohapel, P.; Bouter, B.; Frielingsdorf, H.; Pizzo, D.; Brundin, P.; Erlanson-Albertsson, C. High-fat diet impairs hippocampal neurogenesis in male rats. Eur. J. Neurol. 2006, 13, 1385–1388. [Google Scholar] [CrossRef]
- Hwang, I.K.; Kim, I.Y.; Kim, D.W.; Yoo, K.Y.; Kim, Y.N.; Yi, S.S.; Won, M.; Lee, I.S.; Yoon, Y.S.; Seong, J.K. Strain-specific differences in cell proliferation and differentiation in the dentate gyrus of C57BL/6N and C3H/HeN mice fed a high fat diet. Brain Res. 2008, 1241, 1–6. [Google Scholar] [CrossRef]
- Yi, S.S.; Hwang, I.K.; Yoo, K.Y.; Park, O.K.; Yu, J.; Yan, B.; Kim, I.Y.; Kim, Y.N.; Pai, T.; Song, W.; et al. Effects of treadmill exercise on cell proliferation and differentiation in the subgranular zone of the dentate gyrus in a rat model of type II diabetes. Neurochem. Res. 2009, 34, 1039–1046. [Google Scholar] [CrossRef]
- Stranahan, A.M.; Lee, K.; Martin, B.; Maudsley, S.; Golden, E.; Cutler, R.G.; Mattson, M.P. Voluntary exercise and caloric restriction enhance hippocampal dendritic spine density and BDNF levels in diabetic mice. Hippocampus 2009, 19, 951–961. [Google Scholar] [CrossRef]
- Takeuchi, T.; Duszkiewicz, A.J.; Morris, R.G. The synaptic plasticity and memory hypothesis: Encoding, storage and persistence. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130288. [Google Scholar] [CrossRef]
- Abraham, W.C.; Jones, O.D.; Glanzman, D.L. Is plasticity of synapses the mechanism of long-term memory storage? NPJ Sci. Learn. 2019, 4, 9. [Google Scholar] [CrossRef]
- Porter, D.W.; Kerr, B.D.; Flatt, P.R.; Holscher, C.; Gault, V.A. Four weeks administration of Liraglutide improves memory and learning as well as glycaemic control in mice with high fat dietary-induced obesity and insulin resistance. Diabetes Obes. Metab. 2010, 12, 891–899. [Google Scholar] [CrossRef]
- Gault, V.A.; Porter, W.D.; Flatt, P.R.; Holscher, C. Actions of exendin-4 therapy on cognitive function and hippocampal synaptic plasticity in mice fed a high-fat diet. Int. J. Obes. 2010, 34, 1341–1344. [Google Scholar] [CrossRef] [PubMed]
- Kamal, A.; Biessels, G.J.; Urban, I.J.; Gispen, W.H. Hippocampal synaptic plasticity in streptozotocin-diabetic rats: Impairment of long-term potentiation and facilitation of long-term depression. Neuroscience 1999, 90, 737–745. [Google Scholar] [CrossRef]
- Kamal, A.; Biessels, G.J.; Duis, S.E.; Gispen, W.H. Learning and hippocampal synaptic plasticity in streptozotocin-diabetic rats: Interaction of diabetes and ageing. Diabetologia 2000, 43, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Manschot, S.M.; Biessels, G.J.; Cameron, N.E.; Cotter, M.A.; Kamal, A.; Kappelle, L.J.; Gispen, W.H. Angiotensin converting enzyme inhibition partially prevents deficits in water maze performance, hippocampal synaptic plasticity and cerebral blood flow in streptozotocin-diabetic rats. Brain Res. 2003, 966, 274–282. [Google Scholar] [CrossRef]
- Jing, X.H.; Chen, S.L.; Shi, H.; Cai, H.; Jin, Z.G. Electroacupuncture restores learning and memory impairment induced by both diabetes mellitus and cerebral ischemia in rats. Neurosci. Lett. 2008, 443, 193–198. [Google Scholar] [CrossRef]
- Kamal, A.; Biessels, G.J.; Ramakers, G.M.; Hendrik Gispen, W. The effect of short duration streptozotocin-induced diabetes mellitus on the late phase and threshold of long-term potentiation induction in the rat. Brain Res. 2005, 1053, 126–130. [Google Scholar] [CrossRef]
- Artola, A.; Kamal, A.; Ramakers, G.M.; Gardoni, F.; Di Luca, M.; Biessels, G.J.; Cattabeni, F.; Gispen, W.H. Synaptic plasticity in the diabetic brain: Advanced aging? Prog. Brain Res. 2002, 138, 305–314. [Google Scholar]
- Katagiri, H.; Tanaka, K.; Manabe, T. Requirement of appropriate glutamate concentrations in the synaptic cleft for hippocampal LTP induction. Eur. J. Neurosci. 2001, 14, 547–553. [Google Scholar] [CrossRef]
- Brennan, B.P.; Hudson, J.I.; Jensen, J.E.; McCarthy, J.; Roberts, J.L.; Prescot, A.P.; Cohen, B.M.; Pope, H.G.; Renshaw, P.F.; Ongur, D. Rapid enhancement of glutamatergic neurotransmission in bipolar depression following treatment with riluzole. Neuropsychopharmacology 2010, 35, 834–846. [Google Scholar] [CrossRef]
- Lim, S.I.; Song, K.H.; Yoo, C.H.; Woo, D.C.; Choe, B.Y. High-fat diet-induced hyperglutamatergic activation of the hippocampus in mice: A proton magnetic resonance spectroscopy study at 9.4T. Neurochem. Int. 2018, 114, 10–17. [Google Scholar] [CrossRef]
- Lewerenz, J.; Maher, P. Chronic Glutamate Toxicity in Neurodegenerative Diseases-What is the Evidence? Front. Neurosci. 2015, 9, 469. [Google Scholar] [CrossRef] [PubMed]
- Passafaro, M.; Piech, V.; Sheng, M. Subunit-specific temporal and spatial patterns of AMPA receptor exocytosis in hippocampal neurons. Nat. Neurosci. 2001, 4, 917–926. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, M.; Fusco, S.; Mainardi, M.; Scala, F.; Natale, F.; Lapenta, R.; Mattera, A.; Rinaudo, M.; Puma, D.D.L.; Ripoli, C. Brain insulin resistance impairs hippocampal synaptic plasticity and memory by increasing GluA1 palmitoylation through FoxO3a. Nat. Commun. 2017, 8, 2009. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhong, Z.; Wang, B.; Xia, X.; Yao, W.; Huang, L.; Wang, Y.; Ding, W. Early-life high-fat diet-induced obesity programs hippocampal development and cognitive functions via regulation of gut commensal Akkermansia muciniphila. Neuropsychopharmacology 2019, 44, 2054–2064. [Google Scholar] [CrossRef] [PubMed]
- Valladolid-Acebes, I.; Merino, B.; Principato, A.; Fole, A.; Barbas, C.; Lorenzo, M.P.; Garcia, A.; del Olmo, N.; Ruiz-Gayo, M.; Cano, V. High-fat diets induce changes in hippocampal glutamate metabolism and neurotransmission. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E396–E402. [Google Scholar] [CrossRef]
- Lizarbe, B.; Soares, A.F.; Larsson, S.; Duarte, J.M.N. Neurochemical Modifications in the Hippocampus, Cortex and Hypothalamus of Mice Exposed to Long-Term High-Fat Diet. Front. Neurosci. 2018, 12, 985. [Google Scholar] [CrossRef]
- Berge, L.I.; Riise, T. Comorbidity between Type 2 Diabetes and Depression in the Adult Population: Directions of the Association and Its Possible Pathophysiological Mechanisms. Int. J. Endocrinol. 2015, 2015, 164760. [Google Scholar] [CrossRef]
- Nikolic, M. Prevalence of comorbid depression and obesity in general practice. Br. J. Gen. Pract. 2015, 65, 451. [Google Scholar] [CrossRef]
- Shpilberg, Y.; Beaudry, J.L.; D'Souza, A.; Campbell, J.E.; Peckett, A.; Riddell, M.C. A rodent model of rapid-onset diabetes induced by glucocorticoids and high-fat feeding. Dis. Models Mech. 2012, 5, 671–680. [Google Scholar] [CrossRef]
- Zemdegs, J.; Quesseveur, G.; Jarriault, D.; Penicaud, L.; Fioramonti, X.; Guiard, B.P. High-fat diet-induced metabolic disorders impairs 5-HT function and anxiety-like behavior in mice. Br. J. Pharmacol. 2016, 173, 2095–2110. [Google Scholar] [CrossRef]
- Hassan, A.M.; Mancano, G.; Kashofer, K.; Frohlich, E.E.; Matak, A.; Mayerhofer, R.; Reichmann, F.; Olivares, M.; Neyrinck, A.M.; Delzenne, N.M.; et al. High-fat diet induces depression-like behaviour in mice associated with changes in microbiome, neuropeptide Y, and brain metabolome. Nutr. Neurosci. 2019, 22, 877–893. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.M.; Mancano, G.; Kashofer, K.; Liebisch, G.; Farzi, A.; Zenz, G.; Claus, S.P.; Holzer, P. Anhedonia induced by high-fat diet in mice depends on gut microbiota and leptin. Nutr. Neurosci. 2020, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Abe-Higuchi, N.; Uchida, S.; Yamagata, H.; Higuchi, F.; Hobara, T.; Hara, K.; Kobayashi, A.; Watanabe, Y. Hippocampal Sirtuin 1 Signaling Mediates Depression-like Behavior. Biol. Psychiatry 2016, 80, 815–826. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, H.; Marchisella, F.; Ortega-Martinez, S.; Hollos, P.; Eerola, K.; Komulainen, E.; Kulesskaya, N.; Freemantle, E.; Fagerholm, V.; Savontous, E.; et al. JNK1 controls adult hippocampal neurogenesis and imposes cell-autonomous control of anxiety behaviour from the neurogenic niche. Mol. Psychiatry 2018, 23, 362–374. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.Q.; Hu, Z.L.; Hu, Y.L.; Chen, X.; Wang, J.; Mao, L.; Lu, X.; Ni, M.; Chen, J.; Wang, F. Hippocampal CD39/ENTPD1 promotes mouse depression-like behavior through hydrolyzing extracellular ATP. EMBO Rep. 2020, 21, e47857. [Google Scholar] [CrossRef] [PubMed]
- Bavley, C.C.; Fischer, D.K.; Rizzo, B.K.; Rajadhyaksha, A.M. Cav1.2 channels mediate persistent chronic stress-induced behavioral deficits that are associated with prefrontal cortex activation of the p25/Cdk5-glucocorticoid receptor pathway. Neurobiol. Stress 2017, 7, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Wolf, G.; Lifschytz, T.; Ben-Ari, H.; Tatarskyy, P.; Merzel, T.K.; Lotan, A.; Lerer, B. Effect of chronic unpredictable stress on mice with developmental under-expression of the Ahi1 gene: Behavioral manifestations and neurobiological correlates. Transl. Psychiatry 2018, 8, 124. [Google Scholar] [CrossRef] [PubMed]
- Rudyk, C.; Dwyer, Z.; McNeill, J.; Salmaso, N.; Farmer, K.; Prowse, N.; Hayley, S. Chronic unpredictable stress influenced the behavioral but not the neurodegenerative impact of paraquat. Neurobiol. Stress 2019, 11, 100179. [Google Scholar] [CrossRef]
- Palumbo, M.C.; Dominguez, S.; Dong, H. Sex differences in hypothalamic-pituitary-adrenal axis regulation after chronic unpredictable stress. Brain Behav. 2020, 10, e01586. [Google Scholar] [CrossRef]
- Tannenbaum, B.M.; Brindley, D.N.; Tannenbaum, G.S.; Dallman, M.F.; McArthur, M.D.; Meaney, M.J. High-fat feeding alters both basal and stress-induced hypothalamic-pituitary-adrenal activity in the rat. Am. J. Physiol. 1997, 273, E1168–E1177. [Google Scholar] [CrossRef]
- Swierczynska, M.M.; Mateska, I.; Peitzsch, M.; Bornstein, S.R.; Chavakis, T.; Eisenhofer, G.; Lamounier-Zepter, V.; Eaton, S. Changes in morphology and function of adrenal cortex in mice fed a high-fat diet. Int. J. Obes. 2015, 39, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Sobesky, J.L.; D'Angelo, H.M.; Weber, M.D.; Anderson, N.D.; Frank, M.G.; Watkins, L.R.; Maier, S.F.; Barrientos, R.M. Glucocorticoids Mediate Short-Term High-Fat Diet Induction of Neuroinflammatory Priming, the NLRP3 Inflammasome, and the Danger Signal HMGB1. eNeuro 2016, 3, ENEURO.0113-16.2016. [Google Scholar] [CrossRef] [PubMed]
- Boudaba, C.; Szabo, K.; Tasker, J.G. Physiological mapping of local inhibitory inputs to the hypothalamic paraventricular nucleus. J. Neurosci. 1996, 16, 7151–7160. [Google Scholar] [CrossRef] [PubMed]
- Herman, J.P.; Dolgas, C.M.; Carlson, S.L. Ventral subiculum regulates hypothalamo-pituitary-adrenocortical and behavioural responses to cognitive stressors. Neuroscience 1998, 86, 449–459. [Google Scholar] [CrossRef]
- Herman, J.P.; Tasker, J.G.; Ziegler, D.R.; Cullinan, W.E. Local circuit regulation of paraventricular nucleus stress integration: Glutamate-GABA connections. Pharmacol. Biochem. Behav. 2002, 71, 457–468. [Google Scholar] [CrossRef]
- Yau, S.Y.; Lau, B.W.; Tong, J.B.; Wong, R.; Ching, Y.P.; Qiu, G.; Tang, S.; Lee, T.M.C.; So, K. Hippocampal neurogenesis and dendritic plasticity support running-improved spatial learning and depression-like behaviour in stressed rats. PLoS ONE 2011, 6, e24263. [Google Scholar] [CrossRef]
- Yau, S.Y.; Lau, B.W.; Zhang, E.D.; Lee, J.C.; Li, A.; Lee, T.M.; Ching, Y.; Xu, A.; So, K. Effects of voluntary running on plasma levels of neurotrophins, hippocampal cell proliferation and learning and memory in stressed rats. Neuroscience 2012, 222, 289–301. [Google Scholar] [CrossRef]
- Yau, S.Y.; Li, A.; Zhang, E.D.; Christie, B.R.; Xu, A.; Lee, T.M.; So, K. Sustained running in rats administered corticosterone prevents the development of depressive behaviors and enhances hippocampal neurogenesis and synaptic plasticity without increasing neurotrophic factor levels. Cell Transpl. 2014, 23, 481–492. [Google Scholar] [CrossRef]
- Gainey, S.J.; Kwakwa, K.A.; Bray, J.K.; Pillote, M.M.; Tir, V.L.; Towers, A.E.; Freund, G.G. Short-Term High-Fat Diet (HFD) Induced Anxiety-Like Behaviors and Cognitive Impairment Are Improved with Treatment by Glyburide. Front. Behav. Neurosci. 2016, 10, 156. [Google Scholar] [CrossRef]
- Wang, Z.; Ge, Q.; Wu, Y.; Zhang, J.; Gu, Q.; Han, J. Impairment of Long-term Memory by a Short-term High-fat Diet via Hippocampal Oxidative Stress and Alterations in Synaptic Plasticity. Neuroscience 2020, 424, 24–33. [Google Scholar] [CrossRef]
- McLean, F.H.; Grant, C.; Morris, A.C.; Horgan, G.W.; Polanski, A.J.; Allan, K.; Campbell, F.M.; Langston, R.F.; Williams, L.M. Rapid and reversible impairment of episodic memory by a high-fat diet in mice. Sci. Rep. 2018, 8, 11976. [Google Scholar] [CrossRef] [PubMed]
- Sims-Robinson, C.; Bakeman, A.; Bruno, E.; Jackson, S.; Glasser, R.; Murphy, G.G.; Feldman, E.L. Dietary Reversal Ameliorates Short- and Long-Term Memory Deficits Induced by High-fat Diet Early in Life. PLoS ONE 2016, 11, e0163883. [Google Scholar] [CrossRef] [PubMed]
- Avena, N.M. Examining the addictive-like properties of binge eating using an animal model of sugar dependence. Exp. Clin. Psychopharmacol. 2007, 15, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Valdivia, S.; Patrone, A.; Reynaldo, M.; Perello, M. Acute high fat diet consumption activates the mesolimbic circuit and requires orexin signaling in a mouse model. PLoS ONE 2014, 9, e87478. [Google Scholar] [CrossRef]
- Bodnar, H.; Denyko, B.; Waenke, P.; Ball, K.T. Vulnerability to diet-induced obesity is associated with greater food priming-induced reinstatement of palatable food seeking. Physiol. Behav. 2020, 213, 112730. [Google Scholar] [CrossRef]
- Kumar, M.; Chail, M. Sucrose and saccharin differentially modulate depression and anxiety-like behavior in diabetic mice: Exposures and withdrawal effects. Psychopharmacology 2019, 236, 3095–3110. [Google Scholar] [CrossRef]
- Razzoli, M.; Sanghez, V.; Bartolomucci, A. Chronic subordination stress induces hyperphagia and disrupts eating behavior in mice modeling binge-eating-like disorder. Front. Nutr. 2015, 1, 30. [Google Scholar] [CrossRef]
- Sefton, C.; Harno, E.; Davies, A.; Small, H.; Allen, T.J.; Wray, J.R.; Lawrence, C.B.; Coll, A.P.; White, A. Elevated Hypothalamic Glucocorticoid Levels Are Associated with Obesity and Hyperphagia in Male Mice. Endocrinology 2016, 157, 4257–4265. [Google Scholar] [CrossRef]
- Razzoli, M.; Pearson, C.; Crow, S.; Bartolomucci, A. Stress, overeating, and obesity: Insights from human studies and preclinical models. Neurosci. Biobehav. Rev. 2017, 76 (Pt A), 154–162. [Google Scholar] [CrossRef]
- Henderson, Y.O.; Smith, G.P.; Parent, M.B. Hippocampal neurons inhibit meal onset. Hippocampus 2013, 23, 100–107. [Google Scholar] [CrossRef]
- Henderson, Y.O.; Nalloor, R.; Vazdarjanova, A.; Parent, M.B. Sweet orosensation induces Arc expression in dorsal hippocampal CA1 neurons in an experience-dependent manner. Hippocampus 2016, 26, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Parent, M.B. Cognitive control of meal onset and meal size: Role of dorsal hippocampal-dependent episodic memory. Physiol. Behav. 2016, 162, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Davidson, T.L.; Chan, K.; Jarrard, L.E.; Kanoski, S.E.; Clegg, D.J.; Benoit, S.C. Contributions of the hippocampus and medial prefrontal cortex to energy and body weight regulation. Hippocampus 2009, 19, 235–252. [Google Scholar] [CrossRef]
- Sweeney, P.; Yang, Y. An excitatory ventral hippocampus to lateral septum circuit that suppresses feeding. Nat. Commun. 2015, 6, 10188. [Google Scholar] [CrossRef] [PubMed]
- Kanoski, S.E.; Hayes, M.R.; Greenwald, H.S.; Fortin, S.M.; Gianessi, C.A.; Gilbert, J.R.; Grill, H.J. Hippocampal leptin signaling reduces food intake and modulates food-related memory processing. Neuropsychopharmacology 2011, 36, 1859–1870. [Google Scholar] [CrossRef] [PubMed]
- Hsu, T.M.; Hahn, J.D.; Konanur, V.R.; Lam, A.; Kanoski, S.E. Hippocampal GLP-1 receptors influence food intake, meal size, and effort-based responding for food through volume transmission. Neuropsychopharmacology 2015, 40, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Hannapel, R.C.; Henderson, Y.H.; Nalloor, R.; Vazdarjanova, A.; Parent, M.B. Ventral hippocampal neurons inhibit postprandial energy intake. Hippocampus 2017, 27, 274–284. [Google Scholar] [CrossRef]
- Morin, J.P.; Rodriguez-Duran, L.F.; Guzman-Ramos, K.; Perez-Cruz, C.; Ferreira, G.; Diaz-Cintra, S.; Pacheco-Lopez, G. Palatable Hyper-Caloric Foods Impact on Neuronal Plasticity. Front. Behav. Neurosci. 2017, 11, 19. [Google Scholar] [CrossRef]
- Davidson, T.L.; Jones, S.; Roy, M.; Stevenson, R.J. The Cognitive Control of Eating and Body Weight: It's More Than What You "Think". Front. Psychol. 2019, 10, 62. [Google Scholar] [CrossRef]
- Yau, S.Y.; Li, A.; Hoo, R.L.; Ching, Y.P.; Christie, B.R.; Lee, T.M.; Xu, A.; So, K. Physical exercise-induced hippocampal neurogenesis and antidepressant effects are mediated by the adipocyte hormone adiponectin. Proc. Natl. Acad. Sci. USA 2014, 111, 15810–15815. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, X.; Lu, X.Y. Adiponectin Exerts Neurotrophic Effects on Dendritic Arborization, Spinogenesis, and Neurogenesis of the Dentate Gyrus of Male Mice. Endocrinology 2016, 157, 2853–2869. [Google Scholar] [CrossRef] [PubMed]
- Bloemer, J.; Pinky, P.D.; Smith, W.D.; Bhattacharya, D.; Chauhan, A.; Govindarajulu, M.; Hong, H.; Dhanasekaran, M.; Judd, R.; Amin, R.H.; et al. Adiponectin Knockout Mice Display Cognitive and Synaptic Deficits. Front. Endocrinol. 2019, 10, 819. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Liang, Y.; Chen, K.; Yau, S.Y.; Sun, X.; Cheng, K.K.; Xu, A.; So, K.; Li, A. Potential Involvement of Adiponectin Signaling in Regulating Physical Exercise-Elicited Hippocampal Neurogenesis and Dendritic Morphology in Stressed Mice. Front. Cell. Neurosci. 2020, 14, 189. [Google Scholar] [CrossRef] [PubMed]
- Li, X.L.; Aou, S.; Oomura, Y.; Hori, N.; Fukunaga, K.; Hori, T. Impairment of long-term potentiation and spatial memory in leptin receptor-deficient rodents. Neuroscience 2002, 113, 607–615. [Google Scholar] [CrossRef]
- Winocur, G.; Greenwood, C.E.; Piroli, G.G.; Grillo, C.A.; Reznikov, L.R.; Reagan, L.P.; McEwen, B.S. Memory impairment in obese Zucker rats: An investigation of cognitive function in an animal model of insulin resistance and obesity. Behav. Neurosci. 2005, 119, 1389–1395. [Google Scholar] [CrossRef]
- Oomura, Y.; Hori, N.; Shiraishi, T.; Fukunaga, K.; Takeda, H.; Tsuji, M.; Matsumiya, T.; Ishibashi, M.; Aou, S.; Li, X.L.; et al. Leptin facilitates learning and memory performance and enhances hippocampal CA1 long-term potentiation and CaMK II phosphorylation in rats. Peptides 2006, 27, 2738–2749. [Google Scholar] [CrossRef]
- Yook, J.S.; Rakwal, R.; Shibato, J.; Takahashi, K.; Koizumi, H.; Shima, T.; Ikemoto, M.J.; Oharomari, L.K.; McEwen, B.S.; Soya, H. Leptin in hippocampus mediates benefits of mild exercise by an antioxidant on neurogenesis and memory. Proc. Natl. Acad. Sci. USA 2019, 116, 10988–10993. [Google Scholar] [CrossRef]
- Naranjo, V.; Contreras, A.; Merino, B.; Plaza, A.; Lorenzo, M.P.; Garcia-Caceres, C.; Chowen, J.A.; Ruiz-Gayo, M.; del Olmo, N. Specific Deletion of the Astrocyte Leptin Receptor Induces Changes in Hippocampus Glutamate Metabolism, Synaptic Transmission and Plasticity. Neuroscience 2019, 447, 182–190. [Google Scholar] [CrossRef]
- Dinel, A.L.; Andre, C.; Aubert, A.; Ferreira, G.; Laye, S.; Castanon, N. Cognitive and emotional alterations are related to hippocampal inflammation in a mouse model of metabolic syndrome. PLoS ONE 2011, 6, e24325. [Google Scholar] [CrossRef]
- Boitard, C.; Cavaroc, A.; Sauvant, J.; Aubert, A.; Castanon, N.; Laye, S.; Ferreira, G. Impairment of hippocampal-dependent memory induced by juvenile high-fat diet intake is associated with enhanced hippocampal inflammation in rats. Brain Behav. Immun. 2014, 40, 9–17. [Google Scholar] [CrossRef]
- Arita, Y.; Kihara, S.; Ouchi, N.; Takahashi, M.; Maeda, K.; Miyagawa, J.; Hotta, K.; Shimomura, I.; Nakamura, T.; Miyaoka, K. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem. Biophys. Res. Commun. 1999, 257, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Jansson, P.A.; Pellme, F.; Hammarstedt, A.; Sandqvist, M.; Brekke, H.; Caidahl, K.; Forsberg, M.; Volkmann, R.; Carvalho, E.; Funahashi, T.; et al. A novel cellular marker of insulin resistance and early atherosclerosis in humans is related to impaired fat cell differentiation and low adiponectin. FASEB J. 2003, 17, 1434–1440. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Guo, M.; Zhang, D.; Cheng, S.Y.; Liu, M.; Ding, J.; Scherer, P.E.; Liu, F.; Lu, X. Adiponectin is critical in determining susceptibility to depressive behaviors and has antidepressant-like activity. Proc. Natl. Acad. Sci. USA 2012, 109, 12248–12253. [Google Scholar] [CrossRef] [PubMed]
- Ng, R.C.; Cheng, O.Y.; Jian, M.; Kwan, J.S.; Ho, P.W.; Cheng, K.K.; Yeung, P.K.K.; Zhou, L.L.; Hoo, R.L.; Chung, S.K.; et al. Chronic adiponectin deficiency leads to Alzheimer's disease-like cognitive impairments and pathologies through AMPK inactivation and cerebral insulin resistance in aged mice. Mol. Neurodegener. 2016, 11, 71. [Google Scholar] [CrossRef]
- Guo, M.; Li, C.; Lei, Y.; Xu, S.; Zhao, D.; Lu, X.Y. Role of the adipose PPARgamma-adiponectin axis in susceptibility to stress and depression/anxiety-related behaviors. Mol. Psychiatry 2017, 22, 1056–1068. [Google Scholar] [CrossRef] [PubMed]
- Rege, S.D.; Geetha, T.; Broderick, T.L.; Babu, J.R. Can Diet and Physical Activity Limit Alzheimer's Disease Risk? Curr. Alzheimer Res. 2017, 14, 76–93. [Google Scholar] [CrossRef]
- Heiston, E.M.; Eichner, N.Z.; Gilbertson, N.M.; Malin, S.K. Exercise improves adiposopathy, insulin sensitivity and metabolic syndrome severity independent of intensity. Exp. Physiol. 2020, 105, 632–640. [Google Scholar] [CrossRef]
- Dyer, A.H.; Briggs, R.; Mockler, D.; Gibney, J.; Kennelly, S.P. Non-pharmacological interventions for cognition in patients with Type 2 diabetes mellitus: A systematic review. QJM 2020, 113, 155–161. [Google Scholar] [CrossRef]
- Srikanth, V.; Sinclair, A.J.; Hill-Briggs, F.; Moran, C.; Biessels, G.J. Type 2 diabetes and cognitive dysfunction-towards effective management of both comorbidities. Lancet Diabetes Endocrinol. 2020, 8, 535–545. [Google Scholar] [CrossRef]
- Pereira, S.S.; Alvarez-Leite, J.I. Low-Grade Inflammation, Obesity, and Diabetes. Curr. Obes. Rep. 2014, 3, 422–431. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Ridaura, V.K.; Faith, J.J.; Rey, F.E.; Cheng, J.; Duncan, A.E.; Kau, A.L.; Griffin, N.W.; Lombard, V.; Henrissat, B.; Bain, J.R.; et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 2013, 341, 1241214. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Neyrinck, A.M.; Fava, F.; Knauf, C.; Burcelin, R.G.; Tuohy, K.M.; Gibson, G.R.; Delzenne, N.M. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia 2007, 50, 2374–2383. [Google Scholar] [CrossRef]
- Cani, P.D.; Bibiloni, R.; Knauf, C.; Waget, A.; Neyrinck, A.M.; Delzenne, N.M.; Burcelin, R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 2008, 57, 1470–1481. [Google Scholar] [CrossRef]
- Ji, Y.; Sakata, Y.; Tso, P. Nutrient-induced inflammation in the intestine. Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 315–321. [Google Scholar] [CrossRef]
- Hersoug, L.G.; Moller, P.; Loft, S. Role of microbiota-derived lipopolysaccharide in adipose tissue inflammation, adipocyte size and pyroptosis during obesity. Nutr. Res. Rev. 2018, 31, 153–163. [Google Scholar] [CrossRef]
- Griffiths, E.A.; Duffy, L.C.; Schanbacher, F.L.; Qiao, H.; Dryja, D.; Leavens, A.; Rossman, J.; Rich, G.; Dirienzo, D.; Ogra, P.L. In vivo effects of bifidobacteria and lactoferrin on gut endotoxin concentration and mucosal immunity in Balb/c mice. Dig. Dis. Sci. 2004, 49, 579–589. [Google Scholar] [CrossRef]
- Tsukumo, D.M.; Carvalho-Filho, M.A.; Carvalheira, J.B.; Prada, P.O.; Hirabara, S.M.; Schenka, A.A.; Araujo, E.P.; Vassallo, J.; Curi, R.; Velloso, L.A.; et al. Loss-of-function mutation in Toll-like receptor 4 prevents diet-induced obesity and insulin resistance. Diabetes 2007, 56, 1986–1998. [Google Scholar] [CrossRef]
- Cani, P.D.; Possemiers, S.; Van de Wiele, T.; Guiot, Y.; Everard, A.; Rottier, O.; Geurts, L.; Naslain, D.; Neyrinck, A.; Lambert, D.M.; et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut 2009, 58, 1091–1103. [Google Scholar] [CrossRef]
- Lu, Y.C.; Yeh, W.C.; Ohashi, P.S. LPS/TLR4 signal transduction pathway. Cytokine 2008, 42, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Tesar, B.M.; Walker, W.E.; Goldstein, D.R. Dual signaling of MyD88 and TRIF is critical for maximal TLR4-induced dendritic cell maturation. J. Immunol. 2008, 181, 1849–1858. [Google Scholar] [CrossRef] [PubMed]
- Akhter, N.; Hasan, A.; Shenouda, S.; Wilson, A.; Kochumon, S.; Ali, S.; Tuomilehto, J.; Sindhu, S.; Ahmad, R. TLR4/MyD88 -mediated CCL2 production by lipopolysaccharide (endotoxin): Implications for metabolic inflammation. J. Diabetes Metab. Disord. 2018, 17, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Piao, W.; Ru, L.W.; Piepenbrink, K.H.; Sundberg, E.J.; Vogel, S.N.; Toshchakov, V.Y. Recruitment of TLR adapter TRIF to TLR4 signaling complex is mediated by the second helical region of TRIF TIR domain. Proc. Natl. Acad. Sci. USA 2013, 110, 19036–19041. [Google Scholar] [CrossRef] [PubMed]
- Weisberg, S.P.; Hunter, D.; Huber, R.; Lemieux, J.; Slaymaker, S.; Vaddi, K.; Charo, I.; Leibel, R.L.; Ferrante, A.W., Jr. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J. Clin. Investig. 2006, 116, 115–124. [Google Scholar] [CrossRef]
- Kanda, H.; Tateya, S.; Tamori, Y.; Kotani, K.; Hiasa, K.; Kitazawa, R.; Kitazawa, S.; Miyachi, H.; Maeda, S.; Egashira, K.; et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J. Clin. Investig. 2006, 116, 1494–1505. [Google Scholar] [CrossRef]
- Kamei, N.; Tobe, K.; Suzuki, R.; Ohsugi, M.; Watanabe, T.; Kubota, N.; Ohtsuka-Kowatari, N.; Kumagai, K.; Sakamoto, K.; Kobayashi, M.; et al. Overexpression of monocyte chemoattractant protein-1 in adipose tissues causes macrophage recruitment and insulin resistance. J. Biol. Chem. 2006, 281, 26602–26614. [Google Scholar] [CrossRef]
- Caesar, R.; Tremaroli, V.; Kovatcheva-Datchary, P.; Cani, P.D.; Backhed, F. Crosstalk between Gut Microbiota and Dietary Lipids Aggravates WAT Inflammation through TLR Signaling. Cell Metab. 2015, 22, 658–668. [Google Scholar] [CrossRef]
- Bruce-Keller, A.J.; Salbaum, J.M.; Luo, M.; Blanchard Et Taylor, C.M.; Welsh, D.A.; Berthoud, H. Obese-type gut microbiota induce neurobehavioral changes in the absence of obesity. Biol. Psychiatry 2015, 77, 607–615. [Google Scholar] [CrossRef]
- D'Amato, A.; Di Cesare Mannelli, L.; Lucarini, E.; Man, A.L.; Le Gall, G.; Branca, J.J.V.; Ghelardini, C.; Amedei, A.; Bertelli, E.; Regoli, M.; et al. Faecal microbiota transplant from aged donor mice affects spatial learning and memory via modulating hippocampal synaptic plasticity- and neurotransmission-related proteins in young recipients. Microbiome 2020, 8, 140. [Google Scholar] [CrossRef]
- Kundu, P.; Lee, H.U.; Garcia-Perez, I.; Tay, E.X.Y.; Kim, H.; Faylon, L.E.; Martin, K.A.; Purbojati, R.; Drautz-Moses, D.I.; Ghosh, S.; et al. Neurogenesis and prolongevity signaling in young germ-free mice transplanted with the gut microbiota of old mice. Sci. Transl. Med. 2019, 11, eaau4760. [Google Scholar] [CrossRef] [PubMed]
- Suarez, A.N.; Hsu, T.M.; Liu, C.M.; Noble, E.E.; Cortella, A.M.; Nakamoto, E.M.; Hahn, J.D.; de Lartigue, G.; Kanoski, S.E. Gut vagal sensory signaling regulates hippocampus function through multi-order pathways. Nat. Commun. 2018, 9, 2181. [Google Scholar] [CrossRef] [PubMed]
- Burhans, M.S.; Hagman, D.K.; Kuzma, J.N.; Schmidt, K.A.; Kratz, M. Contribution of Adipose Tissue Inflammation to the Development of Type 2 Diabetes Mellitus. Compr. Physiol. 2018, 9, 1–58. [Google Scholar] [PubMed]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar] [CrossRef] [PubMed]
- Castoldi, A.; Naffah de Souza, C.; Camara, N.O.; Moraes-Vieira, P.M. The Macrophage Switch in Obesity Development. Front. Immunol. 2015, 6, 637. [Google Scholar] [CrossRef]
- Guo, D.H.; Yamamoto, M.; Hernandez, C.M.; Khodadadi, H.; Baban, B.; Stranahan, A.M. Visceral adipose NLRP3 impairs cognition in obesity via IL-1R1 on CX3CR1+ cells. J. Clin. Investig. 2020, 130, 1961–1976. [Google Scholar] [CrossRef]
- Terauchi, Y.; Matsui, J.; Kamon, J.; Yamauchi, T.; Kubota, N.; Komeda, K.; Aizawa, S.; Akanuma, Y.; Tomita, M.; Kadowaki, T. Increased serum leptin protects from adiposity despite the increased glucose uptake in white adipose tissue in mice lacking p85alpha phosphoinositide 3-kinase. Diabetes 2004, 53, 2261–2270. [Google Scholar] [CrossRef]
- Koch, A.; Weiskirchen, R.; Zimmermann, H.W.; Sanson, E.; Trautwein, C.; Tacke, F. Relevance of serum leptin and leptin-receptor concentrations in critically ill patients. Mediat. Inflamm. 2010, 2010, 473540. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Q.; Zhang, L.; Li, C.; Jiang, H. Expression of ghrelin and leptin during the development of type 2 diabetes mellitus in a rat model. Mol. Med. Rep. 2013, 7, 223–228. [Google Scholar] [CrossRef]
- Paglialunga, S.; Ludzki, A.; Root-McCaig, J.; Holloway, G.P. In adipose tissue, increased mitochondrial emission of reactive oxygen species is important for short-term high-fat diet-induced insulin resistance in mice. Diabetologia 2015, 58, 1071–1080. [Google Scholar] [CrossRef]
- Franchi, L.; Eigenbrod, T.; Munoz-Planillo, R.; Nunez, G. The inflammasome: A caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat. Immunol. 2009, 10, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Vandanmagsar, B.; Youm, Y.H.; Ravussin, A.; Galgani, J.E.; Stadler, K.; Mynatt, R.L.; Ravussin, E.; Stephens, J.M.; Dixit, V.D. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat. Med. 2011, 17, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Iyer, S.S.; Pulskens, W.P.; Sadler, J.J.; Butter, L.M.; Teske, G.J.; Ulland, T.K.; Eisenbarth, S.C.; Florquin, S.; Flavell, R.A.; Leemans, J.C.; et al. Necrotic cells trigger a sterile inflammatory response through the Nlrp3 inflammasome. Proc. Natl. Acad. Sci. USA 2009, 106, 20388–20393. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Huang, R.; Han, J.; Cai, R.; Guo, D.; Lin, H.; Wang, J.; Wang, S. Increased plasma Interleukin-1beta level is associated with memory deficits in type 2 diabetic patients with mild cognitive impairment. Psychoneuroendocrinology 2018, 96, 148–154. [Google Scholar] [CrossRef]
- Ouchi, N.; Walsh, K. Adiponectin as an anti-inflammatory factor. Clin. Chim. Acta 2007, 380, 24–30. [Google Scholar] [CrossRef]
- Ouchi, N.; Kihara, S.; Arita, Y.; Maeda, K.; Kuriyama, H.; Okamoto, Y.; Hotta, K.; Nishida, M.; Takahashi, M.; Nakamura, T.; et al. Novel modulator for endothelial adhesion molecules: Adipocyte-derived plasma protein adiponectin. Circulation 1999, 100, 2473–2476. [Google Scholar] [CrossRef]
- Kadowaki, T.; Yamauchi, T.; Kubota, N.; Hara, K.; Ueki, K.; Tobe, K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J. Clin. Investig. 2006, 116, 1784–1792. [Google Scholar] [CrossRef]
- Arnold, S.E.; Lucki, I.; Brookshire, B.R.; Carlson, G.C.; Browne, C.A.; Kazi, H.; Bang, S.; Choi, B.; Chen, Y.; McMullen, M.F.; et al. High fat diet produces brain insulin resistance, synaptodendritic abnormalities and altered behavior in mice. Neurobiol. Dis. 2014, 67, 79–87. [Google Scholar] [CrossRef]
- Qi, Y.; Takahashi, N.; Hileman, S.M.; Patel, H.R.; Berg, A.H.; Pajvani, U.B.; Scherer, P.E.; Ahima, R.S. Adiponectin acts in the brain to decrease body weight. Nat. Med. 2004, 10, 524–529. [Google Scholar] [CrossRef]
- Nicolas, S.; Cazareth, J.; Zarif, H.; Guyon, A.; Heurteaux, C.; Chabry, J.; Petit-Paitel, A. Globular Adiponectin Limits Microglia Pro-Inflammatory Phenotype through an AdipoR1/NF-kappaB Signaling Pathway. Front. Cell. Neurosci. 2017, 11, 352. [Google Scholar] [CrossRef]
- Jeon, B.T.; Jeong, E.A.; Shin, H.J.; Lee, Y.; Lee, D.H.; Kim, H.J.; Kang, S.S.; Cho, G.J.; Choi, W.S.; Roh, G.S. Resveratrol attenuates obesity-associated peripheral and central inflammation and improves memory deficit in mice fed a high-fat diet. Diabetes 2012, 61, 1444–1454. [Google Scholar] [CrossRef] [PubMed]
- Hao, S.; Dey, A.; Yu, X.; Stranahan, A.M. Dietary obesity reversibly induces synaptic stripping by microglia and impairs hippocampal plasticity. Brain Behav. Immun. 2016, 51, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Cope, E.C.; LaMarca, E.A.; Monari, P.K.; Olson, L.B.; Martinez, S.; Zych, A.D.; Katchur, N.J.; Gould, E. Microglia Play an Active Role in Obesity-Associated Cognitive Decline. J. Neurosci. 2018, 38, 8889–8904. [Google Scholar] [CrossRef] [PubMed]
- Castro-Sanchez, S.; Garcia-Yague, A.J.; Kugler, S.; Lastres-Becker, I. CX3CR1-deficient microglia shows impaired signalling of the transcription factor NRF2: Implications in tauopathies. Redox Biol. 2019, 22, 101118. [Google Scholar] [CrossRef] [PubMed]
- Cunnane, S.C.; Trushina, E.; Morland, C.; Prigione, A.; Casadesus, G.; Andrews, Z.B.; Beal, M.F.; Bergersen, L.H.; Brinton, R.D.; de la Monte, S.; et al. Brain energy rescue: An emerging therapeutic concept for neurodegenerative disorders of ageing. Nat. Rev. Drug Discov. 2020, 19, 609–633. [Google Scholar] [CrossRef] [PubMed]
- Heinonen, S.; Buzkova, J.; Muniandy, M.; Kaksonen, R.; Ollikainen, M.; Ismail, K.; Hakkarainen, A.; Lundbom, J.; Lundbom, N.; Vuolteenaho, K.; et al. Impaired Mitochondrial Biogenesis in Adipose Tissue in Acquired Obesity. Diabetes 2015, 64, 3135–3145. [Google Scholar] [CrossRef]
- Bournat, J.C.; Brown, C.W. Mitochondrial dysfunction in obesity. Curr. Opin. Endocrinol. Diabetes Obes. 2010, 17, 446–452. [Google Scholar] [CrossRef]
- Zorzano, A.; Liesa, M.; Palacin, M. Role of mitochondrial dynamics proteins in the pathophysiology of obesity and type 2 diabetes. Int. J. Biochem. Cell Biol. 2009, 41, 1846–1854. [Google Scholar] [CrossRef]
- Nishikawa, T.; Edelstein, D.; Du, X.L.; Yamagishi, S.; Matsumura, T.; Kaneda, Y.; Yorek, M.A.; Beebe, D.; Oates, P.J.; Hammes, H.; et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 2000, 404, 787–790. [Google Scholar] [CrossRef]
- Ruiz-Ramirez, A.; Chavez-Salgado, M.; Peneda-Flores, J.A.; Zapata, E.; Masso, F.; El-Hafidi, M. High-sucrose diet increases ROS generation, FFA accumulation, UCP2 level, and proton leak in liver mitochondria. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E1198–E1207. [Google Scholar] [CrossRef]
- Massaad, C.A.; Klann, E. Reactive oxygen species in the regulation of synaptic plasticity and memory. Antioxid. Redox Signal 2011, 14, 2013–2054. [Google Scholar] [CrossRef] [PubMed]
- Ritz, P.; Berrut, G. Mitochondrial function, energy expenditure, aging and insulin resistance. Diabetes Metab. 2005, 31, 5S67–5S73. [Google Scholar] [CrossRef]
- Zhou, Y.; Lian, S.; Zhang, J.; Lin, D.; Huang, C.; Liu, L.; Chen, Z. Mitochondrial Perturbation Contributing to Cognitive Decline in Streptozotocin-Induced Type 1 Diabetic Rats. Cell. Physiol. Biochem. 2018, 46, 1668–1682. [Google Scholar] [CrossRef] [PubMed]
- Austin, S.; St-Pierre, J. PGC1alpha and mitochondrial metabolism--emerging concepts and relevance in ageing and neurodegenerative disorders. J. Cell. Sci. 2012, 125 (Pt 21), 4963–4971. [Google Scholar] [CrossRef]
- Petrov, D.; Pedros, I.; Artiach, G.; Sureda, F.X.; Barroso, E.; Pallas, M.; Casadesus, G.; Beas-Zarate, C.; Carro, E.; Ferrer, I.; et al. High-fat diet-induced deregulation of hippocampal insulin signaling and mitochondrial homeostasis deficiences contribute to Alzheimer disease pathology in rodents. Biochim. Biophys. Acta 2015, 1852, 1687–1699. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.; Wan, R.; Yang, J.L.; Kamimura, N.; Son, T.G.; Ouyang, X.; Luo, Y.; Okun, E.; Mattson, M.P. Involvement of PGC-1alpha in the formation and maintenance of neuronal dendritic spines. Nat. Commun. 2012, 3, 1250. [Google Scholar] [CrossRef]
- Erturk, A.; Wang, Y.; Sheng, M. Local pruning of dendrites and spines by caspase-3-dependent and proteasome-limited mechanisms. J. Neurosci. 2014, 34, 1672–1688. [Google Scholar] [CrossRef]
- Chen, C.; Wang, Y.; Zhang, J.; Ma, L.; Gu, J.; Ho, G. Contribution of neural cell death to depressive phenotypes of streptozotocin-induced diabetic mice. Dis. Models Mech. 2014, 7, 723–730. [Google Scholar] [CrossRef]
- He, X.; Sun, J.; Huang, X. Expression of caspase-3, Bax and Bcl-2 in hippocampus of rats with diabetes and subarachnoid hemorrhage. Exp. Ther. Med. 2018, 15, 873–877. [Google Scholar] [CrossRef]
- Suzuki, J.; Denning, D.P.; Imanishi, E.; Horvitz, H.R.; Nagata, S. Xk-related protein 8 and CED-8 promote phosphatidylserine exposure in apoptotic cells. Science 2013, 341, 403–406. [Google Scholar] [CrossRef]
- Segawa, K.; Kurata, S.; Yanagihashi, Y.; Brummelkamp, T.R.; Matsuda, F.; Nagata, S. Caspase-mediated cleavage of phospholipid flippase for apoptotic phosphatidylserine exposure. Science 2014, 344, 1164–1168. [Google Scholar] [CrossRef] [PubMed]
- Fuller, A.D.; Van Eldik, L.J. MFG-E8 regulates microglial phagocytosis of apoptotic neurons. J. Neuroimmune Pharmacol. 2008, 3, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Li, X.C.; Hu, Q.K.; Chen, L.; Liu, S.Y.; Su, S.; Tao, H.; Zhang, L.; Sun, T.; He, L. HSPB8 Promotes the Fusion of Autophagosome and Lysosome during Autophagy in Diabetic Neurons. Int. J. Med. Sci. 2017, 14, 1335–1341. [Google Scholar] [CrossRef] [PubMed]
- Sangwung, P.; Petersen, K.F.; Shulman, G.I.; Knowles, J.W. Mitochondrial Dysfunction, Insulin Resistance, and Potential Genetic Implications. Endocrinology 2020, 161, bqaa017. [Google Scholar] [CrossRef] [PubMed]
- Iwabu, M.; Yamauchi, T.; Okada-Iwabu, M.; Sato, K.; Nakagawa, T.; Funata, M.; Yamaguchi, M.; Namiki, S.; Nakayama, R.; Tabata, M.; et al. Adiponectin and AdipoR1 regulate PGC-1alpha and mitochondria by Ca(2+) and AMPK/SIRT1. Nature 2010, 464, 1313–1319. [Google Scholar] [CrossRef] [PubMed]
- Pepin, M.E.; Koentges, C.; Pfeil, K.; Gollmer, J.; Kersting, S.; Wiese, S.; Hoffman, M.M.; Odening, K.; Muehlen, C.v.; Diehl, P.; et al. Dysregulation of the Mitochondrial Proteome Occurs in Mice Lacking Adiponectin Receptor 1. Front. Endocrinol. 2019, 10, 872. [Google Scholar] [CrossRef]
- Piccinin, E.; Villani, G.; Moschetta, A. Metabolic aspects in NAFLD, NASH and hepatocellular carcinoma: The role of PGC1 coactivators. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 160–174. [Google Scholar] [CrossRef]
- Liu, B.; Liu, J.; Wang, J.G.; Liu, C.L.; Yan, H.J. AdipoRon improves cognitive dysfunction of Alzheimer's disease and rescues impaired neural stem cell proliferation through AdipoR1/AMPK pathway. Exp. Neurol. 2020, 327, 113249. [Google Scholar] [CrossRef]
- Kim, M.W.; Abid, N.B.; Jo, M.H.; Jo, M.G.; Yoon, G.H.; Kim, M.O. Suppression of adiponectin receptor 1 promotes memory dysfunction and Alzheimer's disease-like pathologies. Sci. Rep. 2017, 7, 12435. [Google Scholar] [CrossRef]
- Quehenberger, O.; Armando, A.M.; Brown, A.H.; Milne, S.B.; Myers, D.S.; Merrill, A.H.; Bandyopadhyay, S.; Jones, K.N.; Kelly, S.; Shaner, R.L. Lipidomics reveals a remarkable diversity of lipids in human plasma. J. Lipid Res. 2010, 51, 3299–3305. [Google Scholar] [CrossRef]
- Pilitsis, J.G.; Diaz, F.G.; Wellwood, J.M.; Oregan, M.H.; Fairfax, M.R.; Phillis, J.W.; Coplin, W.M. Quantification of free fatty acids in human cerebrospinal fluid. Neurochem. Res. 2001, 26, 1265–1270. [Google Scholar] [CrossRef] [PubMed]
- Karmi, A.; Iozzo, P.; Viljanen, A.; Hirvonen, J.; Fielding, B.A.; Virtanen, K.; Oikonen, V.; Kemppainen, J.; Viljanen, T.; Guiducci, L.; et al. Increased brain fatty acid uptake in metabolic syndrome. Diabetes 2010, 59, 2171–2177. [Google Scholar] [CrossRef] [PubMed]
- Melo, H.M.; Seixas da Silva, G.D.S.; Sant'Ana, M.R.; Teixeira, C.V.L.; Clarke, J.R.; Miya Coreixas, V.S.; de Melo, B.C.; Fortuna, J.T.S.; Forny-Germano, L.; Ledo, J.H.; et al. Palmitate Is Increased in the Cerebrospinal Fluid of Humans with Obesity and Induces Memory Impairment in Mice via Pro-inflammatory TNF-alpha. Cell Rep. 2020, 30, 2180–2194. [Google Scholar] [CrossRef] [PubMed]
- Vinuesa, A.; Bentivegna, M.; Calfa, G.; Filipello, F.; Pomilio, C.; Bonaventura, M.M.; Lux-Lantos, V.; Matzkin, M.E.; Gregosa, A.; Presa, J.; et al. Early Exposure to a High-Fat Diet Impacts on Hippocampal Plasticity: Implication of Microglia-Derived Exosome-like Extracellular Vesicles. Mol. Neurobiol. 2019, 56, 5075–5094. [Google Scholar] [CrossRef]
- Steffensen, C.; Dekkers, O.M.; Lyhne, J.; Pedersen, B.G.; Rasmussen, F.; Rungby, J.; Poulsen, P.L.; Jorgensen, J.O.L. Hypercortisolism in Newly Diagnosed Type 2 Diabetes: A Prospective Study of 384 Newly Diagnosed Patients. Horm. Metab. Res. 2019, 51, 62–68. [Google Scholar] [CrossRef]
- Buchenauer, T.; Behrendt, P.; Bode, F.J.; Horn, R.; Brabant, G.; Stephan, M.; Nave, H. Diet-induced obesity alters behavior as well as serum levels of corticosterone in F344 rats. Physiol. Behav. 2009, 98, 563–569. [Google Scholar] [CrossRef]
- Wosiski-Kuhn, M.; Erion, J.R.; Gomez-Sanchez, E.P.; Gomez-Sanchez, C.E.; Stranahan, A.M. Glucocorticoid receptor activation impairs hippocampal plasticity by suppressing BDNF expression in obese mice. Psychoneuroendocrinology 2014, 42, 165–177. [Google Scholar] [CrossRef]
- Oster, H.; Challet, E.; Ott, V.; Arvat, E.; de Kloet, E.R.; Dijk, D.J.; Lightman, S.; Vgontzas, A.; van Cauter, E. The Functional and Clinical Significance of the 24-Hour Rhythm of Circulating Glucocorticoids. Endocr. Rev. 2017, 38, 3–45. [Google Scholar] [CrossRef]
- Herman, J.P.; Schafer, M.K.; Young, E.A.; Thompson, R.; Douglass, J.; Akil, H.; Watson, S.J. Evidence for hippocampal regulation of neuroendocrine neurons of the hypothalamo-pituitary-adrenocortical axis. J. Neurosci. 1989, 9, 3072–3082. [Google Scholar] [CrossRef]
- Song, Y.; Meng, Q.X.; Wu, K.; Hua, R.; Song, Z.J.; Song, Y.; Qin, X.; Cao, J.; Zhang, Y. Disinhibition of PVN-projecting GABAergic neurons in AV region in BNST participates in visceral hypersensitivity in rats. Psychoneuroendocrinology 2020, 117, 104690. [Google Scholar] [CrossRef]
- Herman, J.P. Regulation of adrenocorticosteroid receptor mRNA expression in the central nervous system. Cell Mol. Neurobiol. 1993, 13, 349–372. [Google Scholar] [CrossRef] [PubMed]
- Mason, B.L.; Pariante, C.M.; Jamel, S.; Thomas, S.A. Central nervous system (CNS) delivery of glucocorticoids is fine-tuned by saturable transporters at the blood-CNS barriers and nonbarrier regions. Endocrinology 2010, 151, 5294–5305. [Google Scholar] [CrossRef] [PubMed]
- Boyle, M.P.; Kolber, B.J.; Vogt, S.K.; Wozniak, D.F.; Muglia, L.J. Forebrain glucocorticoid receptors modulate anxiety-associated locomotor activation and adrenal responsiveness. J. Neurosci. 2006, 26, 1971–1978. [Google Scholar] [CrossRef] [PubMed]
- Furay, A.R.; Bruestle, A.E.; Herman, J.P. The role of the forebrain glucocorticoid receptor in acute and chronic stress. Endocrinology 2008, 149, 5482–5490. [Google Scholar] [CrossRef]
- Check, J.H.; Wilson, C.; Cohen, R.; Sarumi, M. Evidence that Mifepristone, a progesterone receptor antagonist, can cross the blood brain barrier and provide palliative benefits for glioblastoma multiforme grade IV. Anticancer Res. 2014, 34, 2385–2388. [Google Scholar]
- Brummelte, S.; Galea, L.A. Chronic high corticosterone reduces neurogenesis in the dentate gyrus of adult male and female rats. Neuroscience 2010, 168, 680–690. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Y.; Wang, Z. Chronic corticosterone exposure reduces hippocampal astrocyte structural plasticity and induces hippocampal atrophy in mice. Neurosci. Lett. 2015, 592, 76–81. [Google Scholar] [CrossRef]
- Park, H.J.; Lee, S.; Jung, J.W.; Kim, B.C.; Ryu, J.H.; Kim, D.H. Glucocorticoid- and long-term stress-induced aberrant synaptic plasticity are mediated by activation of the glucocorticoid receptor. Arch. Pharm. Res. 2015, 38, 1204–1212. [Google Scholar] [CrossRef]
- Khazen, T.; Hatoum, O.A.; Ferreira, G.; Maroun, M. Acute exposure to a high-fat diet in juvenile male rats disrupts hippocampal-dependent memory and plasticity through glucocorticoids. Sci. Rep. 2019, 9, 12270. [Google Scholar] [CrossRef]
- Michel, V.; Peinnequin, A.; Alonso, A.; Buguet, A.; Cespuglio, R.; Canini, F. Effect of glucocorticoid depletion on heat-induced Hsp70, IL-1beta and TNF-alpha gene expression. Brain Res. 2007, 1164, 63–71. [Google Scholar] [CrossRef]
- Vilela, F.C.; Antunes-Rodrigues, J.; Elias, L.L.; Giusti-Paiva, A. Corticosterone synthesis inhibitor metyrapone preserves changes in maternal behavior and neuroendocrine responses during immunological challenge in lactating rats. Neuroendocrinology 2013, 97, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Dey, A.; Hao, S.; Erion, J.R.; Wosiski-Kuhn, M.; Stranahan, A.M. Glucocorticoid sensitization of microglia in a genetic mouse model of obesity and diabetes. J. Neuroimmunol. 2014, 269, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Stranahan, A.M.; Arumugam, T.V.; Mattson, M.P. Lowering corticosterone levels reinstates hippocampal brain-derived neurotropic factor and Trkb expression without influencing deficits in hypothalamic brain-derived neurotropic factor expression in leptin receptor-deficient mice. Neuroendocrinology 2011, 93, 58–64. [Google Scholar] [CrossRef]
- Binder, D.K.; Scharfman, H.E. Brain-derived neurotrophic factor. Growth Factors 2004, 22, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Chao, M.V.; Bothwell, M.A.; Ross, A.H.; Koprowski, H.; Lanahan, A.A.; Buck, C.R.; Sehgal, A. Gene transfer and molecular cloning of the human NGF receptor. Science 1986, 232, 518–521. [Google Scholar] [CrossRef] [PubMed]
- Zagrebelsky, M.; Holz, A.; Dechant, G.; Barde, Y.A.; Bonhoeffer, T.; Korte, M. The p75 neurotrophin receptor negatively modulates dendrite complexity and spine density in hippocampal neurons. J. Neurosci. 2005, 25, 9989–9999. [Google Scholar] [CrossRef] [PubMed]
- Paoli, A. Ketogenic diet for obesity: Friend or foe? Int. J. Environ. Res. Public Health 2014, 11, 2092–2107. [Google Scholar] [CrossRef] [PubMed]
- Ulamek-Koziol, M.; Czuczwar, S.J.; Januszewski, S.; Pluta, R. Ketogenic Diet and Epilepsy. Nutrients 2019, 11, 2510. [Google Scholar] [CrossRef]
- Manninen, A.H. Metabolic effects of the very-low-carbohydrate diets: Misunderstood "villains" of human metabolism. J. Int. Soc. Sports Nutr. 2004, 1, 7–11. [Google Scholar] [CrossRef]
- Dhatariya, K.K.; Glaser, N.S.; Codner, E.; Umpierrez, G.E. Diabetic ketoacidosis. Nat. Rev. Dis. Primers 2020, 6, 40. [Google Scholar] [CrossRef]
- Cahill, G.F., Jr.; Herrera, M.G.; Morgan, A.P.; Soeldner, J.S.; Steinke, J.; Levy, P.L.; Reichard, G.A.; Kipnis, D.M. Hormone-fuel interrelationships during fasting. J. Clin. Investig. 1966, 45, 1751–1769. [Google Scholar] [CrossRef] [PubMed]
- Adrogue, H.J.; Wilson, H.; Boyd, A.E., 3rd; Suki, W.N.; Eknoyan, G. Plasma acid-base patterns in diabetic ketoacidosis. N. Engl. J. Med. 1982, 307, 1603–1610. [Google Scholar] [CrossRef] [PubMed]
- Kanikarla-Marie, P.; Jain, S.K. Hyperketonemia and ketosis increase the risk of complications in type 1 diabetes. Free Radic. Biol. Med. 2016, 95, 268–277. [Google Scholar] [CrossRef]
- Veech, R.L.; Chance, B.; Kashiwaya, Y.; Lardy, H.A.; Cahill, G.F., Jr. Ketone bodies, potential therapeutic uses. IUBMB Life 2001, 51, 241–247. [Google Scholar] [PubMed]
- Neal, E.G.; Chaffe, H.; Schwartz, R.H.; Lawson, M.S.; Edwards, N.; Fitzsimmons, G.; Whitney, A.; Cross, J.H. A randomized trial of classical and medium-chain triglyceride ketogenic diets in the treatment of childhood epilepsy. Epilepsia 2009, 50, 1109–1117. [Google Scholar] [CrossRef]
- Achanta, L.B.; Rae, C.D. beta-Hydroxybutyrate in the Brain: One Molecule, Multiple Mechanisms. Neurochem. Res. 2017, 42, 35–49. [Google Scholar] [CrossRef]
- Goldberg, E.L.; Shchukina, I.; Asher, J.L.; Sidorov, S.; Artyomov, M.N.; Dixit, V.D. Ketogenesis activates metabolically protective gammadelta T cells in visceral adipose tissue. Nat. Metab. 2020, 2, 50–61. [Google Scholar] [CrossRef]
- Asrih, M.; Altirriba, J.; Rohner-Jeanrenaud, F.; Jornayvaz, F.R. Ketogenic Diet Impairs FGF21 Signaling and Promotes Differential Inflammatory Responses in the Liver and White Adipose Tissue. PLoS ONE 2015, 10, e0126364. [Google Scholar] [CrossRef]
- Newman, J.C.; Covarrubias, A.J.; Zhao, M.; Yu, X.; Gut, P.; Ng, C.P.; Huang, Y.; Haldar, S.; Verdin, E. Ketogenic Diet Reduces Midlife Mortality and Improves Memory in Aging Mice. Cell Metab. 2017, 26, 547–557. [Google Scholar] [CrossRef]
- Elamin, M.; Ruskin, D.N.; Masino, S.A.; Sacchetti, P. Ketogenic Diet Modulates NAD(+)-Dependent Enzymes and Reduces DNA Damage in Hippocampus. Front. Cell. Neurosci. 2018, 12, 263. [Google Scholar] [CrossRef]
- Hasan-Olive, M.M.; Lauritzen, K.H.; Ali, M.; Rasmussen, L.J.; Storm-Mathisen, J.; Bergersen, L.H. A Ketogenic Diet Improves Mitochondrial Biogenesis and Bioenergetics via the PGC1alpha-SIRT3-UCP2 Axis. Neurochem. Res. 2019, 44, 22–37. [Google Scholar] [CrossRef] [PubMed]
- Belenky, P.; Bogan, K.L.; Brenner, C. NAD+ metabolism in health and disease. Trends Biochem. Sci. 2007, 32, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Murata, M.M.; Kong, X.; Moncada, E.; Chen, Y.; Imamura, H.; Wang, P.; Berns, M.W.; Yokomori, K.; Digman, M.A. NAD+ consumption by PARP1 in response to DNA damage triggers metabolic shift critical for damaged cell survival. Mol. Biol. Cell 2019, 30, 2584–2597. [Google Scholar] [CrossRef] [PubMed]
- Yeung, F.; Hoberg, J.E.; Ramsey, C.S.; Keller, M.D.; Jones, D.R.; Frye, R.A.; Mayo, M.W. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004, 23, 2369–2380. [Google Scholar] [CrossRef] [PubMed]
- Kauppinen, A.; Suuronen, T.; Ojala, J.; Kaarniranta, K.; Salminen, A. Antagonistic crosstalk between NF-kappaB and SIRT1 in the regulation of inflammation and metabolic disorders. Cell Signal 2013, 25, 1939–1948. [Google Scholar] [CrossRef] [PubMed]
- Cohen, H.Y.; Miller, C.; Bitterman, K.J.; Wall, N.R.; Hekking, B.; Kessler, B.; Howitz, K.T.; Gorospe, M.; de Cabo, R.; Sinclair, D.A. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science 2004, 305, 390–392. [Google Scholar] [CrossRef]
- Kim, D.; Nguyen, M.D.; Dobbin, M.M.; Fischer, A.; Sananbenesi, F.; Rodgers, J.T.; Delalle, I.; Baur, J.A.; Sui, G.; Armour, S.M.; et al. SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer's disease and amyotrophic lateral sclerosis. EMBO J. 2007, 26, 3169–3179. [Google Scholar] [CrossRef]
- Khan, R.S.; Fonseca-Kelly, Z.; Callinan, C.; Zuo, L.; Sachdeva, M.M.; Shindler, K.S. SIRT1 activating compounds reduce oxidative stress and prevent cell death in neuronal cells. Front. Cell. Neurosci. 2012, 6, 63. [Google Scholar] [CrossRef]
- Lauritzen, K.H.; Hasan-Olive, M.M.; Regnell, C.E.; Kleppa, L.; Scheibye-Knudsen, M.; Gjedde, A.; Klungland, A.; Bohr, V.A.; Storm-Mathisen, J.; Bergersen, L.H. A ketogenic diet accelerates neurodegeneration in mice with induced mitochondrial DNA toxicity in the forebrain. Neurobiol. Aging 2016, 48, 34–47. [Google Scholar] [CrossRef]
- Kane, A.E.; Sinclair, D.A. Sirtuins and NAD(+) in the Development and Treatment of Metabolic and Cardiovascular Diseases. Circ. Res. 2018, 123, 868–885. [Google Scholar] [CrossRef]
- Vaziri, H.; Dessain, S.K.; Ng Eaton, E.; Imai, S.I.; Frye, R.A.; Pandita, T.K.; Guarente, L.; Weinberg, R.A. hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell 2001, 107, 149–159. [Google Scholar] [CrossRef]
- Mouchiroud, L.; Houtkooper, R.H.; Moullan, N.; Katsyuba, E.; Ryu, D.; Canto, C.; Mottis, A.; Jo, Y.; Viswanathan, M.; Schoonjans, K.; et al. The NAD(+)/Sirtuin Pathway Modulates Longevity through Activation of Mitochondrial UPR and FOXO Signaling. Cell 2013, 154, 430–441. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, J.T.; Lerin, C.; Haas, W.; Gygi, S.P.; Spiegelman, B.M.; Puigserver, P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature 2005, 434, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Rajamohan, S.B.; Pillai, V.B.; Gupta, M.; Sundaresan, N.R.; Birukov, K.G.; Samant, S.; Hottiger, M.O.; Gupta, M.P. SIRT1 promotes cell survival under stress by deacetylation-dependent deactivation of poly(ADP-ribose) polymerase 1. Mol. Cell Biol. 2009, 29, 4116–4129. [Google Scholar] [CrossRef] [PubMed]
- Hirschey, M.D.; Shimazu, T.; Goetzman, E.; Jing, E.; Schwer, B.; Lombard, D.B.; Grueter, C.A.; Harris, C.; Biddinger, S.; Ilkayeva, O.R.; et al. SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature 2010, 464, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Nogueiras, R.; Habegger, K.M.; Chaudhary, N.; Finan, B.; Banks, A.S.; Dietrich, M.O.; Horvath, T.L.; Sinclair, D.A.; Pfluger, P.T.; Tschop, M.H. Sirtuin 1 and sirtuin 3: Physiological modulators of metabolism. Physiol. Rev. 2012, 92, 1479–1514. [Google Scholar] [CrossRef] [PubMed]
- Carnevale, I.; Pellegrini, L.; D'Aquila, P.; Saladini, S.; Lococo, E.; Polletta, L.; Vernucci, E.; Foglio, E.; Coppola, S.; Sansone, L.; et al. SIRT1-SIRT3 Axis Regulates Cellular Response to Oxidative Stress and Etoposide. J. Cell. Physiol. 2017, 232, 1835–1844. [Google Scholar] [CrossRef]
- Chen, D.; Steele, A.D.; Lindquist, S.; Guarente, L. Increase in activity during calorie restriction requires Sirt1. Science 2005, 310, 1641. [Google Scholar] [CrossRef]
- Bordone, L.; Cohen, D.; Robinson, A.; Motta, M.C.; van Veen, E.; Czopik, A.; Steele, A.D.; Crowe, H.; Marmor, S.; Luo, J. SIRT1 transgenic mice show phenotypes resembling calorie restriction. Aging Cell 2007, 6, 759–767. [Google Scholar] [CrossRef]
- Schenk, S.; McCurdy, C.E.; Philp, A.; Chen, M.Z.; Holliday, M.J.; Bandyopadhyay, G.K.; Osborn, O.; Baar, K.; Olefsky, J.M. Sirt1 enhances skeletal muscle insulin sensitivity in mice during caloric restriction. J. Clin. Investig. 2011, 121, 4281–4288. [Google Scholar] [CrossRef]
- Chen, D.; Bruno, J.; Easlon, E.; Lin, S.J.; Cheng, H.L.; Alt, F.W.; Guarente, L. Tissue-specific regulation of SIRT1 by calorie restriction. Genes Dev. 2008, 22, 1753–1757. [Google Scholar] [CrossRef] [PubMed]
- Heyward, F.D.; Gilliam, D.; Coleman, M.A.; Gavin, C.F.; Wang, J.; Kaas, G.; Trieu, R.; Lewis, J.; Moulden, J.; Sweatt, J.D. Obesity Weighs down Memory through a Mechanism Involving the Neuroepigenetic Dysregulation of Sirt1. J. Neurosci. 2016, 36, 1324–1335. [Google Scholar] [CrossRef] [PubMed]
- Fusco, S.; Ripoli, C.; Podda, M.V.; Ranieri, S.C.; Leone, L.; Toietta, G.; McBurney, M.W.; Schutz, G.; Riccio, A.; Grassi, C.; et al. A role for neuronal cAMP responsive-element binding (CREB)-1 in brain responses to calorie restriction. Proc. Natl. Acad. Sci. USA 2012, 109, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.; Seok, S.; Yau, P.; Li, X.; Kemper, B.; Kemper, J.K. Obesity and aging diminish sirtuin 1 (SIRT1)-mediated deacetylation of SIRT3, leading to hyperacetylation and decreased activity and stability of SIRT3. J. Biol. Chem. 2017, 292, 17312–17323. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cheng, A.; Li, Y.-J.; Yang, Y.; Kishimoto, Y.; Zhang, S.; Wang, Y.; Wan, R.; Raefsky, S.M.; Lu, D.; et al. SIRT3 mediates hippocampal synaptic adaptations to intermittent fasting and ameliorates deficits in APP mutant mice. Nat. Commun. 2019, 10, 1886. [Google Scholar] [CrossRef]
- Fu, J.; Jin, J.; Cichewicz, R.H.; Hageman, S.A.; Ellis, T.K.; Xiang, L.; Peng, Q.; Jiang, M.; Arbez, N.; Hotaling, K.; et al. Trans-(-)-epsilon-Viniferin increases mitochondrial sirtuin 3 (SIRT3), activates AMP-activated protein kinase (AMPK), and protects cells in models of Huntington Disease. J. Biol. Chem. 2012, 287, 24460–24472. [Google Scholar] [CrossRef]
- Liu, Z.; Dai, X.; Zhang, H.; Shi, R.; Hui, Y.; Jin, X.; Zhang, W.; Wang, L.; Wang, Q.; Wang, D.; et al. Gut microbiota mediates intermittent-fasting alleviation of diabetes-induced cognitive impairment. Nat. Commun. 2020, 11, 855. [Google Scholar] [CrossRef]
- Aleidi, S.; Issa, A.; Bustanji, H.; Khalil, M.; Bustanji, Y. Adiponectin serum levels correlate with insulin resistance in type 2 diabetic patients. Saudi Pharm. J. 2015, 23, 250–256. [Google Scholar] [CrossRef]
- Gariballa, S.; Alkaabi, J.; Yasin, J.; Al Essa, A. Total adiponectin in overweight and obese subjects and its response to visceral fat loss. BMC Endocr. Disord. 2019, 19, 55. [Google Scholar] [CrossRef]
- Gauthier, M.S.; Couturier, K.; Latour, J.G.; Lavoie, J.M. Concurrent exercise prevents high-fat-diet-induced macrovesicular hepatic steatosis. J. Appl. Physiol. 2003, 94, 2127–2134. [Google Scholar] [CrossRef]
- Gauthier, M.S.; Couturier, K.; Charbonneau, A.; Lavoie, J.M. Effects of introducing physical training in the course of a 16-week high-fat diet regimen on hepatic steatosis, adipose tissue fat accumulation, and plasma lipid profile. Int. J. Obes. Relat. Metab. Disord. 2004, 28, 1064–1071. [Google Scholar] [CrossRef] [PubMed]
- Bradley, R.L.; Jeon, J.Y.; Liu, F.F.; Maratos-Flier, E. Voluntary exercise improves insulin sensitivity and adipose tissue inflammation in diet-induced obese mice. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E586–E594. [Google Scholar] [CrossRef] [PubMed]
- Maesako, M.; Uemura, K.; Kubota, M.; Kuzuya, A.; Sasaki, K.; Hayashida, N.; Asada-Utsugi, M.; Watanabe, K.; Uemura, M.; Kihara, T.; et al. Exercise is more effective than diet control in preventing high fat diet-induced beta-amyloid deposition and memory deficit in amyloid precursor protein transgenic mice. J. Biol. Chem. 2012, 287, 23024–23033. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Jung, S.Y.; Kim, K.; Kim, C.J. Treadmill exercise ameliorates Alzheimer disease-associated memory loss through the Wnt signaling pathway in the streptozotocin-induced diabetic rats. J. Exerc. Rehabil. 2016, 12, 276–283. [Google Scholar] [CrossRef]
- Kim, T.W.; Choi, H.H.; Chung, Y.R. Treadmill exercise alleviates impairment of cognitive function by enhancing hippocampal neuroplasticity in the high-fat diet-induced obese mice. J. Exerc. Rehabil. 2016, 12, 156–162. [Google Scholar] [CrossRef]
- Klein, C.; Jonas, W.; Iggena, D.; Empl, L.; Rivalan, M.; Wiedmer, P.; Spranger, J.; Hellweg, R.; Winter, Y.; Steiner, B. Exercise prevents high-fat diet-induced impairment of flexible memory expression in the water maze and modulates adult hippocampal neurogenesis in mice. Neurobiol. Learn Mem. 2016, 131, 26–35. [Google Scholar] [CrossRef]
- De Senna, P.N.; Bagatini, P.B.; Galland, F.; Bobermin, L.; do Nascimento, P.S.; Nardin, P.; Tramontina, A.C.; Gonccalves, C.A.; Achaval, M.; Xavier, L.L. Physical exercise reverses spatial memory deficit and induces hippocampal astrocyte plasticity in diabetic rats. Brain Res. 2017, 1655, 242–251. [Google Scholar] [CrossRef]
- Shima, T.; Matsui, T.; Jesmin, S.; Okamoto, M.; Soya, M.; Inoue, K.; Liu, Y.; Torres-Aleman, I.; McEwen, B.S.; Soya, H. Moderate exercise ameliorates dysregulated hippocampal glycometabolism and memory function in a rat model of type 2 diabetes. Diabetologia 2017, 60, 597–606. [Google Scholar] [CrossRef]
- Park, H.S.; Cho, H.S.; Kim, T.W. Physical exercise promotes memory capability by enhancing hippocampal mitochondrial functions and inhibiting apoptosis in obesity-induced insulin resistance by high fat diet. Metab. Brain Dis. 2018, 33, 283–292. [Google Scholar] [CrossRef]
- Mehta, B.K.; Singh, K.K.; Banerjee, S. Effect of exercise on type 2 diabetes-associated cognitive impairment in rats. Int. J. Neurosci. 2019, 129, 252–263. [Google Scholar] [CrossRef]
- Wang, Q.; Hu, J.; Liu, Y.; Li, J.; Liu, B.; Li, M.; Lou, S. Aerobic Exercise Improves Synaptic-Related Proteins of Diabetic Rats by Inhibiting FOXO1/NF-kappaB/NLRP3 Inflammatory Signaling Pathway and Ameliorating PI3K/Akt Insulin Signaling Pathway. J. Mol. Neurosci. 2019, 69, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Graham, L.C.; Grabowska, W.A.; Chun, Y.; Risacher, S.L.; Philip, V.M.; Saykin, A.J.; Rizzo, S.J.S.; Howell, G.R. Exercise prevents obesity-induced cognitive decline and white matter damage in mice. Neurobiol. Aging 2019, 80, 154–172. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.W.; Baek, K.W.; Yu, H.S.; Ko, I.G.; Hwang, L.; Park, J.J. High-intensity exercise improves cognitive function and hippocampal brain-derived neurotrophic factor expression in obese mice maintained on high-fat diet. J. Exerc. Rehabil. 2020, 16, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhai, X.; Li, H.; Ji, L. Depression-like behaviors in mice subjected to co-treatment of high-fat diet and corticosterone are ameliorated by AICAR and exercise. J. Affect Disord. 2014, 156, 171–177. [Google Scholar] [CrossRef]
- Cordeira, J.; Monahan, D. Voluntary wheel running reduces weight gain in mice by decreasing high-fat food consumption. Physiol. Behav. 2019, 207, 1–6. [Google Scholar] [CrossRef]
- Liang, N.C.; Bello, N.T.; Moran, T.H. Wheel running reduces high-fat diet intake, preference and mu-opioid agonist stimulated intake. Behav. Brain Res. 2015, 284, 1–10. [Google Scholar] [CrossRef]
- Moody, L.; Liang, J.; Choi, P.P.; Moran, T.H.; Liang, N.C. Wheel running decreases palatable diet preference in Sprague-Dawley rats. Physiol. Behav. 2015, 150, 53–63. [Google Scholar] [CrossRef]
- Woo, J.; Shin, K.O.; Park, S.Y.; Jang, K.S.; Kang, S. Effects of exercise and diet change on cognition function and synaptic plasticity in high fat diet induced obese rats. Lipids Health Dis. 2013, 12, 144. [Google Scholar] [CrossRef]
- Cheng, J.; Chen, L.; Han, S.; Qin, L.; Chen, N.; Wan, Z. Treadmill Running and Rutin Reverse High Fat Diet Induced Cognitive Impairment in Diet Induced Obese Mice. J. Nutr. Health Aging 2016, 20, 503–508. [Google Scholar] [CrossRef]
- Spencer, S.J.; D'Angelo, H.; Soch, A.; Watkins, L.R.; Maier, S.F.; Barrientos, R.M. High-fat diet and aging interact to produce neuroinflammation and impair hippocampal- and amygdalar-dependent memory. Neurobiol. Aging 2017, 58, 88–101. [Google Scholar] [CrossRef]
- De Senna, P.N.; Ilha, J.; Baptista, P.P.; do Nascimento, P.S.; Leite, M.C.; Paim, M.F.; Gonccalves, C.A.; Achaval, M.; Xavier, L.L. Effects of physical exercise on spatial memory and astroglial alterations in the hippocampus of diabetic rats. Metab. Brain Dis. 2011, 26, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Jung, S.Y.; Kim, T.W.; Lee, K.S.; Kim, K. Treadmill exercise decreases incidence of Alzheimer's disease by suppressing glycogen synthase kinase-3beta expression in streptozotocin-induced diabetic rats. J. Exerc. Rehabil. 2015, 11, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Osellame, L.D.; Blacker, T.S.; Duchen, M.R. Cellular and molecular mechanisms of mitochondrial function. Best Pract. Res. Clin. Endocrinol. Metab. 2012, 26, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Boucher, J.; Masri, B.; Daviaud, D.; Gesta, S.; Guigne, C.; Mazzucotelli, A.; Castan-Laurell, I.; Tack, I.; Knibiehler, B.; Carpene, C.; et al. Apelin, a newly identified adipokine up-regulated by insulin and obesity. Endocrinology 2005, 146, 1764–1771. [Google Scholar] [CrossRef]
- Vinel, C.; Lukjanenko, L.; Batut, A.; Deleruyelle, S.; Pradere, J.P.; Le Gonidec, S.; Dortignac, A.; Geoffre, N.; Pereira, O.; Karaz, S.; et al. The exerkine apelin reverses age-associated sarcopenia. Nat. Med. 2018, 24, 1360–1371. [Google Scholar] [CrossRef]
- Yamamoto, T.; Habata, Y.; Matsumoto, Y.; Yasuhara, Y.; Hashimoto, T.; Hamajyo, H.; Anayama, H.; Fujii, R.; Fuse, H.; Shintani, Y.; et al. Apelin-transgenic mice exhibit a resistance against diet-induced obesity by increasing vascular mass and mitochondrial biogenesis in skeletal muscle. Biochim. Biophys. Acta 2011, 1810, 853–862. [Google Scholar] [CrossRef]
- Xu, J.; Jackson, C.W.; Khoury, N.; Escobar, I.; Perez-Pinzon, M.A. Brain SIRT1 Mediates Metabolic Homeostasis and Neuroprotection. Front. Endocrinol. 2018, 9, 702. [Google Scholar] [CrossRef]
- Fan, J.; Guang, H.; Zhang, H.; Chen, D.; Ding, L.; Fan, X.; Gan, Z.; Wang, Y.; Mao, S. SIRT1 Mediates Apelin-13 in Ameliorating Chronic Normobaric Hypoxia-induced Anxiety-like Behavior by Suppressing NF-kappaB Pathway in Mice Hippocampus. Neuroscience 2018, 381, 22–34. [Google Scholar] [CrossRef]
- Gomez-Pinilla, F.; Ying, Z. Differential effects of exercise and dietary docosahexaenoic acid on molecular systems associated with control of allostasis in the hypothalamus and hippocampus. Neuroscience 2010, 168, 130–137. [Google Scholar] [CrossRef]
- Lin, J.Y.; Kuo, W.W.; Baskaran, R.; Kuo, C.H.; Chen, Y.A.; Chen, W.S.; Ho, T.; Day, C.H.; Mahalakshmi, B.; Huang, C. Swimming exercise stimulates IGF1/ PI3K/Akt and AMPK/SIRT1/PGC1alpha survival signaling to suppress apoptosis and inflammation in aging hippocampus. Aging 2020, 12, 6852–6864. [Google Scholar] [CrossRef]
- De Mota, N.; Lenkei, Z.; Llorens-Cortes, C. Cloning, pharmacological characterization and brain distribution of the rat apelin receptor. Neuroendocrinology 2000, 72, 400–407. [Google Scholar] [CrossRef]
- Zhou, S.; Guo, X.; Chen, S.; Xu, Z.; Duan, W.; Zeng, B. Apelin-13 regulates LPS-induced N9 microglia polarization involving STAT3 signaling pathway. Neuropeptides 2019, 76, 101938. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Q.; Yan, Z.Y.; Lan, F.J.; Dong, Y.Q.; Xiong, Y. Suppression of NLRP3 inflammasome attenuates stress-induced depression-like behavior in NLGN3-deficient mice. Biochem. Biophys. Res. Commun. 2018, 501, 933–940. [Google Scholar] [CrossRef] [PubMed]
- Geng, J.; Wei, M.; Yuan, X.; Liu, Z.; Wang, X.; Zhang, D.; Luo, L.; Wu, J.; Guo, W.; Qin, Z. TIGAR regulates mitochondrial functions through SIRT1-PGC1alpha pathway and translocation of TIGAR into mitochondria in skeletal muscle. FASEB J. 2019, 33, 6082–6098. [Google Scholar] [CrossRef] [PubMed]
- Vila, L.; Roca, C.; Elias, I.; Casellas, A.; Lage, R.; Franckhauser, S.; Bosch, F. AAV-mediated Sirt1 overexpression in skeletal muscle activates oxidative capacity but does not prevent insulin resistance. Mol. Ther. Methods Clin. Dev. 2016, 5, 16072. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.J.; Yu, S.P.; Zhang, L.; Wei, L. Neuroprotective effect of the endogenous neural peptide apelin in cultured mouse cortical neurons. Exp. Cell Res. 2010, 316, 1773–1783. [Google Scholar] [CrossRef]
- Bostrom, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Bostrom, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef]
- Jedrychowski, M.P.; Wrann, C.D.; Paulo, J.A.; Gerber, K.K.; Szpyt, J.; Robinson, M.M.; Nair, K.S.; Gygi, S.P.; Spiegelman, B.M. Detection and Quantitation of Circulating Human Irisin by Tandem Mass Spectrometry. Cell Metab. 2015, 22, 734–740. [Google Scholar] [CrossRef]
- Xiong, X.Q.; Chen, D.; Sun, H.J.; Ding, L.; Wang, J.J.; Chen, Q.; Li, Y.; Zhou, Y.; Han, Y.; Zhang, F.; et al. FNDC5 overexpression and irisin ameliorate glucose/lipid metabolic derangements and enhance lipolysis in obesity. Biochim. Biophys. Acta. 2015, 1852, 1867–1875. [Google Scholar] [CrossRef]
- Wrann, C.D.; White, J.P.; Salogiannnis, J.; Laznik-Bogoslavski, D.; Wu, J.; Ma, D.; Lin, J.; Greenberg, M.; Spiegelman, B. Exercise induces hippocampal BDNF through a PGC-1alpha/FNDC5 pathway. Cell Metab. 2013, 18, 649–659. [Google Scholar] [CrossRef]
- Mohammadi, S.; Oryan, S.; Komaki, A.; Eidi, A.; Zarei, M. Effects of Hippocampal Microinjection of Irisin, an Exercise-Induced Myokine, on Spatial and Passive Avoidance Learning and Memory in Male Rats. Int. J. Pept. Res. Ther. 2020, 26, 357–367. [Google Scholar] [CrossRef]
- Mohammadi, S.; Oryan, S.; Komaki, A.; Eidi, A.; Zarei, M. Effects of intra-dentate gyrus microinjection of myokine irisin on long-term potentiation in male rats. Arq. Neuropsiquiatr. 2019, 77, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Yan, S.; Luo, L.; Yang, L. Irisin regulates the expression of BDNF and glycometabolism in diabetic rats. Mol. Med. Rep. 2019, 19, 1074–1082. [Google Scholar] [CrossRef]
- Lourenco, M.V.; Frozza, R.L.; de Freitas, G.B.; Zhang, H.; Kincheski, G.C.; Ribeiro, F.C.; Gonccalves, R.A.; Clarke, J.R.; Beckman, D.; Staniszewski, A.; et al. Exercise-linked FNDC5/irisin rescues synaptic plasticity and memory defects in Alzheimer's models. Nat. Med. 2019, 25, 165–175. [Google Scholar] [CrossRef]
- El Hayek, L.; Khalifeh, M.; Zibara, V.; Abi Assaad, R.; Emmanuel, N.; Karnib, N.; El-Ghandour, R.; Nasrallah, P.; Bilen, M.; Ibrahim, P.; et al. Lactate Mediates the Effects of Exercise on Learning and Memory through SIRT1-Dependent Activation of Hippocampal Brain-Derived Neurotrophic Factor (BDNF). J. Neurosci. 2019, 39, 2369–2382. [Google Scholar] [CrossRef]
- Siteneski, A.; Olescowicz, G.; Pazini, F.L.; Camargo, A.; Fraga, D.B.; Brocardo, P.S.; Gil-Mohapel, J.; Cunha, M.P.; Rodrigues, A.L.S. Antidepressant-like and pro-neurogenic effects of physical exercise: The putative role of FNDC5/irisin pathway. J. Neural Transm. 2020, 127, 355–370. [Google Scholar] [CrossRef]
- Lev-Vachnish, Y.; Cadury, S.; Rotter-Maskowitz, A.; Feldman, N.; Roichman, A.; Illouz, T.; Varvak, A.; Nicola, R.; Madar, R.; Okun, E. L-Lactate Promotes Adult Hippocampal Neurogenesis. Front. Neurosci. 2019, 13, 403. [Google Scholar] [CrossRef]
- Pierre, K.; Pellerin, L. Monocarboxylate transporters in the central nervous system: Distribution, regulation and function. J Neurochem. 2005, 94, 1–14. [Google Scholar] [CrossRef]
- EL, L.J.; Selfridge, J.E.; Burns, J.M.; Swerdlow, R.H. Lactate administration reproduces specific brain and liver exercise-related changes. J. Neurochem. 2013, 127, 91–100. [Google Scholar]
- Hadzic, A.; Nguyen, T.D.; Hosoyamada, M.; Tomioka, N.H.; Bergersen, L.H.; Storm-Mathisen, J.; Morland, C. The Lactate Receptor HCA1 Is Present in the Choroid Plexus, the Tela Choroidea, and the Neuroepithelial Lining of the Dorsal Part of the Third Ventricle. Int. J. Mol. Sci. 2020, 21, 6457. [Google Scholar] [CrossRef]
- Bozzo, L.; Puyal, J.; Chatton, J.Y. Lactate modulates the activity of primary cortical neurons through a receptor-mediated pathway. PLoS ONE 2013, 8, e71721. [Google Scholar] [CrossRef] [PubMed]
- Morland, C.; Andersson, K.A.; Haugen, O.P.; Hadzic, A.; Kleppa, L.; Gille, A.; Rinholm, J.E.; Palibrk, V.; Diget, E.H.; Kennedy, L.H.; et al. Exercise induces cerebral VEGF and angiogenesis via the lactate receptor HCAR1. Nat. Commun. 2017, 8, 15557. [Google Scholar] [CrossRef] [PubMed]
- Carrard, A.; Elsayed, M.; Margineanu, M.; Boury-Jamot, B.; Fragniere, L.; Meylan, E.M.; Petit, J.M.; Fiumelli, H.; Magistretti Pierre, J.; Martin, J.L. Peripheral administration of lactate produces antidepressant-like effects. Mol. Psychiatry 2018, 23, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, Z.; Castano, O.; Castells, A.A.; Mateos-Timoneda, M.A.; Planell, J.A.; Engel, E.; Alcantara, S. Neurogenesis and vascularization of the damaged brain using a lactate-releasing biomimetic scaffold. Biomaterials 2014, 35, 4769–4781. [Google Scholar] [CrossRef]
- Herrera-Lopez, G.; Griego, E.; Galvan, E.J. Lactate induces synapse-specific potentiation on CA3 pyramidal cells of rat hippocampus. PLoS ONE 2020, 15, e0242309. [Google Scholar] [CrossRef]
- Brooks, G.A. The Science and Translation of Lactate Shuttle Theory. Cell Metab. 2018, 27, 757–785. [Google Scholar] [CrossRef]
- Cali, C.; Tauffenberger, A.; Magistretti, P. The Strategic Location of Glycogen and Lactate: From Body Energy Reserve to Brain Plasticity. Front. Cell. Neurosci. 2019, 13, 82. [Google Scholar] [CrossRef]
- Mason, S. Lactate Shuttles in Neuroenergetics-Homeostasis, Allostasis and Beyond. Front. Neurosci. 2017, 11, 43. [Google Scholar] [CrossRef]
- Magistretti, P.J.; Allaman, I. Lactate in the brain: From metabolic end-product to signalling molecule. Nat. Rev. Neurosci. 2018, 19, 235–249. [Google Scholar] [CrossRef]
- Barros, L.F.; Weber, B. CrossTalk proposal: An important astrocyte-to-neuron lactate shuttle couples neuronal activity to glucose utilisation in the brain. J. Physiol. 2018, 596, 347–350. [Google Scholar] [CrossRef]
- Powell, C.L.; Davidson, A.R.; Brown, A.M. Universal Glia to Neurone Lactate Transfer in the Nervous System: Physiological Functions and Pathological Consequences. Biosensors 2020, 10, 183. [Google Scholar] [CrossRef] [PubMed]
- Pierre, K.; Magistretti, P.J.; Pellerin, L. MCT2 is a major neuronal monocarboxylate transporter in the adult mouse brain. J. Cereb. Blood Flow Metab. 2002, 22, 586–595. [Google Scholar] [CrossRef] [PubMed]
- Lucas, S.J.; Michel, C.B.; Marra, V.; Smalley, J.L.; Hennig, M.H.; Graham, B.P.; Forsythe, I.D. Glucose and lactate as metabolic constraints on presynaptic transmission at an excitatory synapse. J. Physiol. 2018, 596, 1699–1721. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, S.; Gharagozloo, M.; Simard, C.; Gris, D. Astrocytes Maintain Glutamate Homeostasis in the CNS by Controlling the Balance between Glutamate Uptake and Release. Cells 2019, 8, 184. [Google Scholar] [CrossRef]
- Allen, A.; Messier, C. Plastic changes in the astrocyte GLUT1 glucose transporter and beta-tubulin microtubule protein following voluntary exercise in mice. Behav. Brain Res. 2013, 240, 95–102. [Google Scholar] [CrossRef]
- Swanson, R.A.; Benington, J.H. Astrocyte glucose metabolism under normal and pathological conditions in vitro. Dev. Neurosci. 1996, 18, 515–521. [Google Scholar] [CrossRef]
- Diaz-Garcia, C.M.; Yellen, G. Neurons rely on glucose rather than astrocytic lactate during stimulation. J. Neurosci. Res. 2019, 97, 883–889. [Google Scholar] [CrossRef]
- Netzahualcoyotzi, C.; Pellerin, L. Neuronal and astroglial monocarboxylate transporters play key but distinct roles in hippocampus-dependent learning and memory formation. Prog. Neurobiol. 2020, 194, 101888. [Google Scholar] [CrossRef]
- Newman, J.C.; Verdin, E. beta-Hydroxybutyrate: A Signaling Metabolite. Annu. Rev. Nutr. 2017, 37, 51–76. [Google Scholar] [CrossRef]
- Laffel, L. Ketone bodies: A review of physiology, pathophysiology and application of monitoring to diabetes. Diabetes Metab. Res. Rev. 1999, 15, 412–426. [Google Scholar] [CrossRef]
- Marosi, K.; Kim, S.W.; Moehl, K.; Scheibye-Knudsen, M.; Cheng, A.; Cutler, R.; Camandola, S.; Mattson, M.P. 3-Hydroxybutyrate regulates energy metabolism and induces BDNF expression in cerebral cortical neurons. J. Neurochem. 2016, 139, 769–781. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.P.; Li, S.N.; Wang, J.F.; Li, Y.; Xie, S.S.; Xue, W.J.; Liu, H.; Huang, B.; Lv, Q.; Lei, L.; et al. BHBA suppresses LPS-induced inflammation in BV-2 cells by inhibiting NF-kappaB activation. Mediat. Inflamm. 2014, 2014, 983401. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Zhu, W.; Lu, M.; Ni, B.; Yang, J. D-beta-hydroxybutyrate promotes functional recovery and relieves pain hypersensitivity in mice with spinal cord injury. Br. J. Pharmacol. 2017, 174, 1961–1971. [Google Scholar] [CrossRef] [PubMed]
- Yamanashi, T.; Iwata, M.; Kamiya, N.; Tsunetomi, K.; Kajitani, N.; Wada, N.; Iitsuka, T.; Yamauchi, T.; Miura, A.; Pu, S.; et al. Beta-hydroxybutyrate, an endogenic NLRP3 inflammasome inhibitor, attenuates stress-induced behavioral and inflammatory responses. Sci. Rep. 2017, 7, 7677. [Google Scholar] [CrossRef]
- Zou, X.H.; Li, H.M.; Wang, S.; Leski, M.; Yao, Y.C.; Yang, X.D.; Huang, Q.; Chen, G. The effect of 3-hydroxybutyrate methyl ester on learning and memory in mice. Biomaterials 2009, 30, 1532–1541. [Google Scholar] [CrossRef]
- Zhang, J.; Cao, Q.; Li, S.; Lu, X.; Zhao, Y.; Guan, J.S.; Chen, J.; Wu, Q.; Chen, G. 3-Hydroxybutyrate methyl ester as a potential drug against Alzheimer's disease via mitochondria protection mechanism. Biomaterials 2013, 34, 7552–7562. [Google Scholar] [CrossRef] [PubMed]
- Sleiman, S.F.; Henry, J.; Al-Haddad, R.; El Hayek, L.; Abou Haidar, E.; Stringer, T.; Ulja, D.; Karuppagounder, S.S.; Holson, E.B.; Ratan, R.R.; et al. Exercise promotes the expression of brain derived neurotrophic factor (BDNF) through the action of the ketone body beta-hydroxybutyrate. Elife 2016, 5, e15092. [Google Scholar] [CrossRef] [PubMed]
- Murray, A.J.; Knight, N.S.; Cole, M.A.; Cochlin, L.E.; Carter, E.; Tchabanenko, K.; Pichulik, T.; Gulston, M.K.; Atherton, H.J.; Schroeder, M.A.; et al. Novel ketone diet enhances physical and cognitive performance. FASEB J. 2016, 30, 4021–4032. [Google Scholar] [CrossRef] [PubMed]
Figure 1. The high-fat diet promotes gut dysbiosis and adipose tissue inflammation. ① The chronic high-fat diet consumption upset the gut microbiome with an increased ratio of gram-negative bacteria to gram-positive bacteria. ② Endotoxemia reduces endothelium integrity, which allows the leakage of endotoxin, such as lipopolysaccharides. ③ LPS-induced activation of TLR4/Trif/MyD88 promotes macrophage recruitment through the secretion of CCL2. ④ Meanwhile, the activation of TLR4 also promotes NRLP3 and pro IL-1β syntheses, facilitating caspase 1-mediated IL-1β formation. ⑤ In obesity, adipose tissue macrophages further infiltrate and accumulate in the fat tissue results in chronic, low-grade inflammation, while ⑥ adiponectin synthesis and secretion are reduced. Created with BioRender.com.
Figure 1. The high-fat diet promotes gut dysbiosis and adipose tissue inflammation. ① The chronic high-fat diet consumption upset the gut microbiome with an increased ratio of gram-negative bacteria to gram-positive bacteria. ② Endotoxemia reduces endothelium integrity, which allows the leakage of endotoxin, such as lipopolysaccharides. ③ LPS-induced activation of TLR4/Trif/MyD88 promotes macrophage recruitment through the secretion of CCL2. ④ Meanwhile, the activation of TLR4 also promotes NRLP3 and pro IL-1β syntheses, facilitating caspase 1-mediated IL-1β formation. ⑤ In obesity, adipose tissue macrophages further infiltrate and accumulate in the fat tissue results in chronic, low-grade inflammation, while ⑥ adiponectin synthesis and secretion are reduced. Created with BioRender.com.
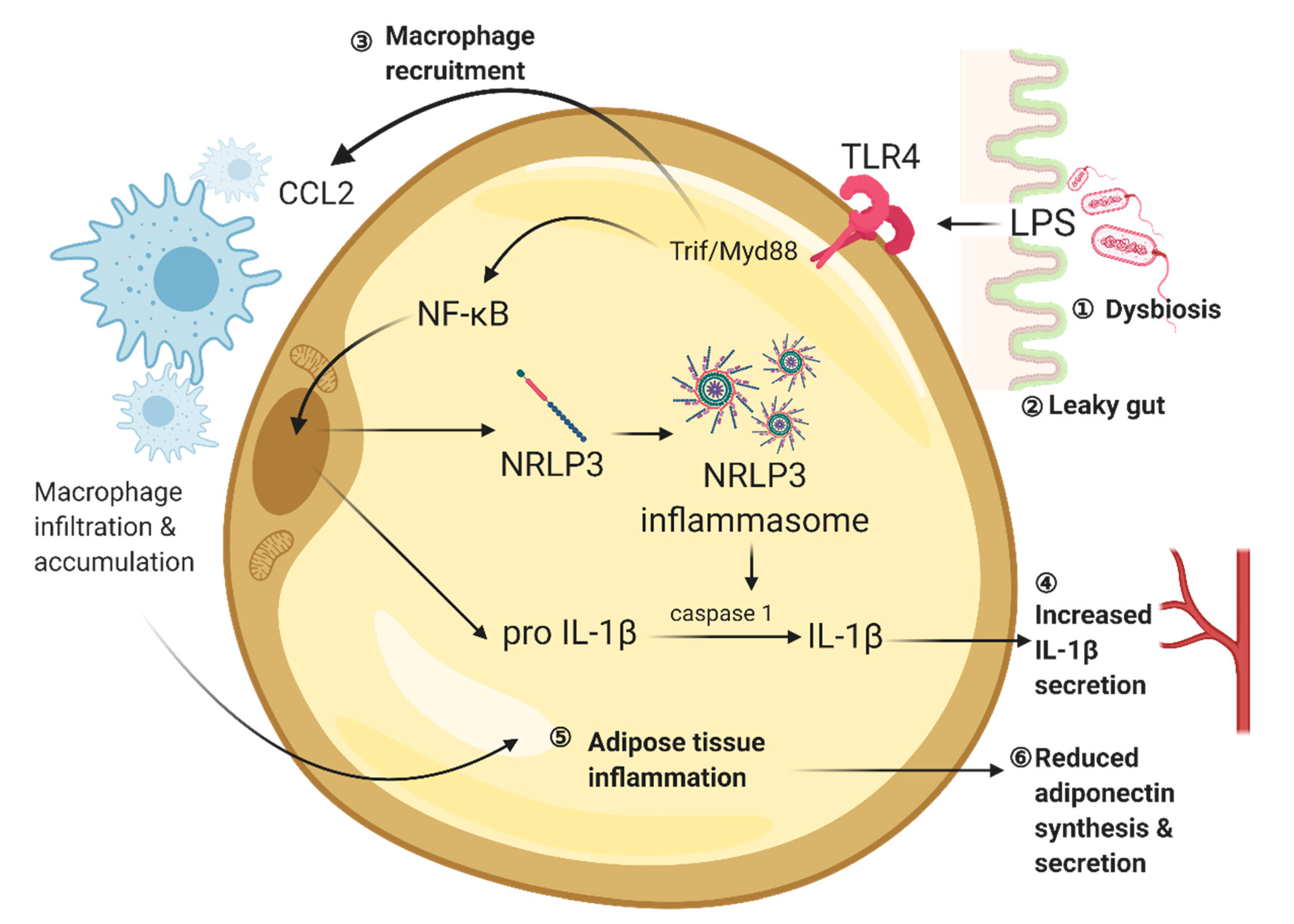
Figure 2. Intrinsic aberrant microglial activity and apoptotic spines can trigger synaptic stripping. ① HFD-induced hippocampal IL-1β elevation promotes the pro-inflammatory M1 phenotype in microglia. ② The aberrant microglia are attracted by CX3CL1 (Fractalkine) from the dendritic spine, which is recognized by the microglial CX3CR1. On the other hand, ③ reduced adiponectin level induces the accumulation of β-amyloid in the hippocampus. ④ Aβ accumulation causes mitochondrial dysfunction with the reduced mitochondrial membrane potential as well as the increased oxidative stress. The apoptotic mitochondrial releases cytochrome c which can activate flippase activity through caspase 3. ⑤ Phosphatidylserine located at the outer membrane of the phospholipid bilayer are served as an 'eat-me' signal, which is recognized by the receptors on the microglia. ⑥ Together, both aberrant microglial activity and malfunctioned neuronal mitochondria can trigger synaptic stripping by the microglia. Created with BioRender.com.
Figure 2. Intrinsic aberrant microglial activity and apoptotic spines can trigger synaptic stripping. ① HFD-induced hippocampal IL-1β elevation promotes the pro-inflammatory M1 phenotype in microglia. ② The aberrant microglia are attracted by CX3CL1 (Fractalkine) from the dendritic spine, which is recognized by the microglial CX3CR1. On the other hand, ③ reduced adiponectin level induces the accumulation of β-amyloid in the hippocampus. ④ Aβ accumulation causes mitochondrial dysfunction with the reduced mitochondrial membrane potential as well as the increased oxidative stress. The apoptotic mitochondrial releases cytochrome c which can activate flippase activity through caspase 3. ⑤ Phosphatidylserine located at the outer membrane of the phospholipid bilayer are served as an 'eat-me' signal, which is recognized by the receptors on the microglia. ⑥ Together, both aberrant microglial activity and malfunctioned neuronal mitochondria can trigger synaptic stripping by the microglia. Created with BioRender.com.
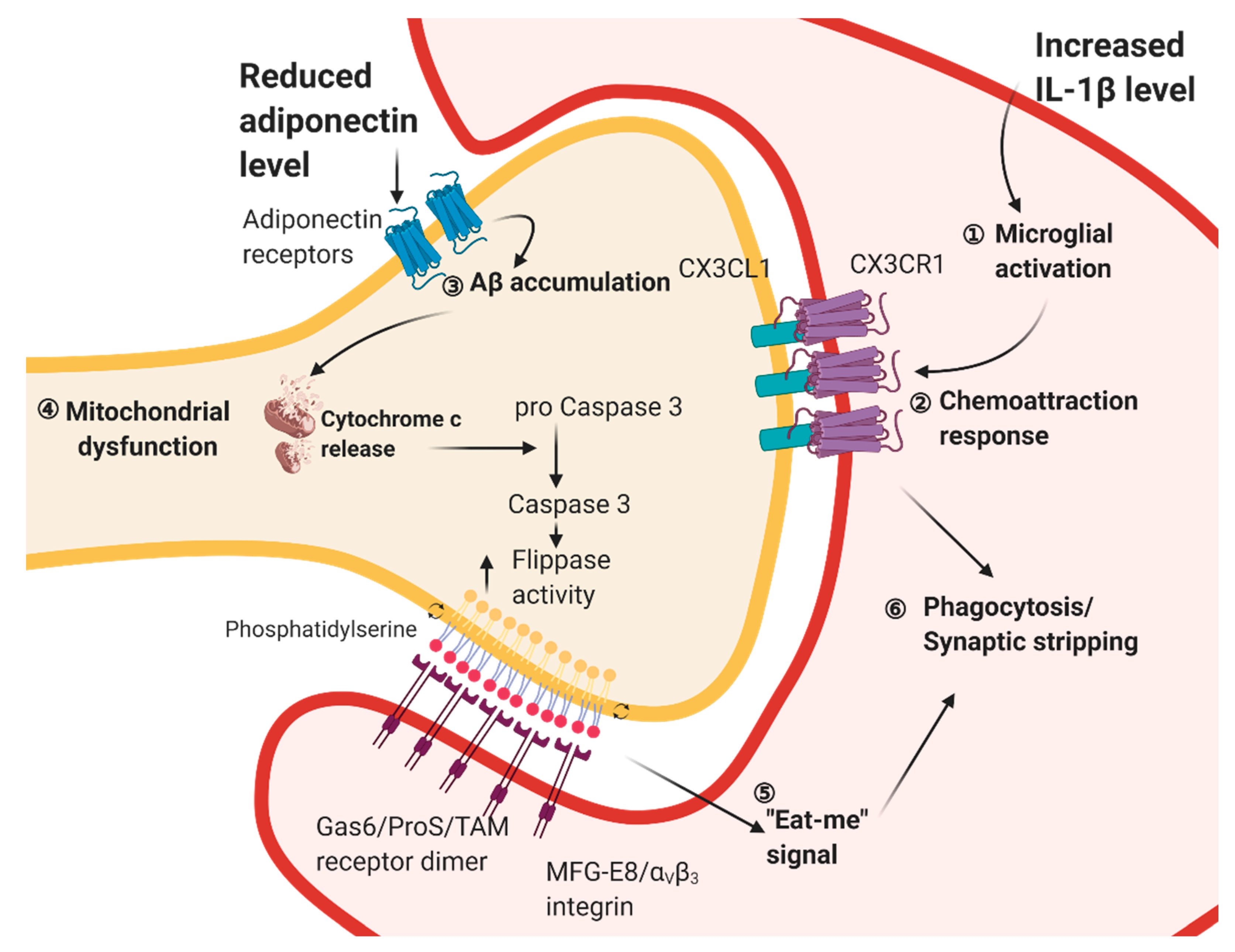
Figure 3. Obese and diabetic conditions disinhibit the hippocampal inhibitory control over the HPA axis. ① HPA axis hyperactivation is reported in diet-induced obese and diabetic conditions, resulting in ② increased corticosterone synthesis and secretion. Corticosterone can cross the blood-brain barrier and activates the glucocorticoid receptors in the hippocampus. The activation of the glucocorticoid receptor ③ impairs neuroplasticity and ④ promotes neuroinflammatory response. ⑤ The hippocampus is shown to inhibit the HPA activity through the vHipp/BNST/PVN neural circuit pathway, whereas dysregulated hippocampal plasticity may disinhibit the HPA axis and aggravates the diet-induced hyperactivation of the HPA axis. Created with BioRender.com.
Figure 3. Obese and diabetic conditions disinhibit the hippocampal inhibitory control over the HPA axis. ① HPA axis hyperactivation is reported in diet-induced obese and diabetic conditions, resulting in ② increased corticosterone synthesis and secretion. Corticosterone can cross the blood-brain barrier and activates the glucocorticoid receptors in the hippocampus. The activation of the glucocorticoid receptor ③ impairs neuroplasticity and ④ promotes neuroinflammatory response. ⑤ The hippocampus is shown to inhibit the HPA activity through the vHipp/BNST/PVN neural circuit pathway, whereas dysregulated hippocampal plasticity may disinhibit the HPA axis and aggravates the diet-induced hyperactivation of the HPA axis. Created with BioRender.com.
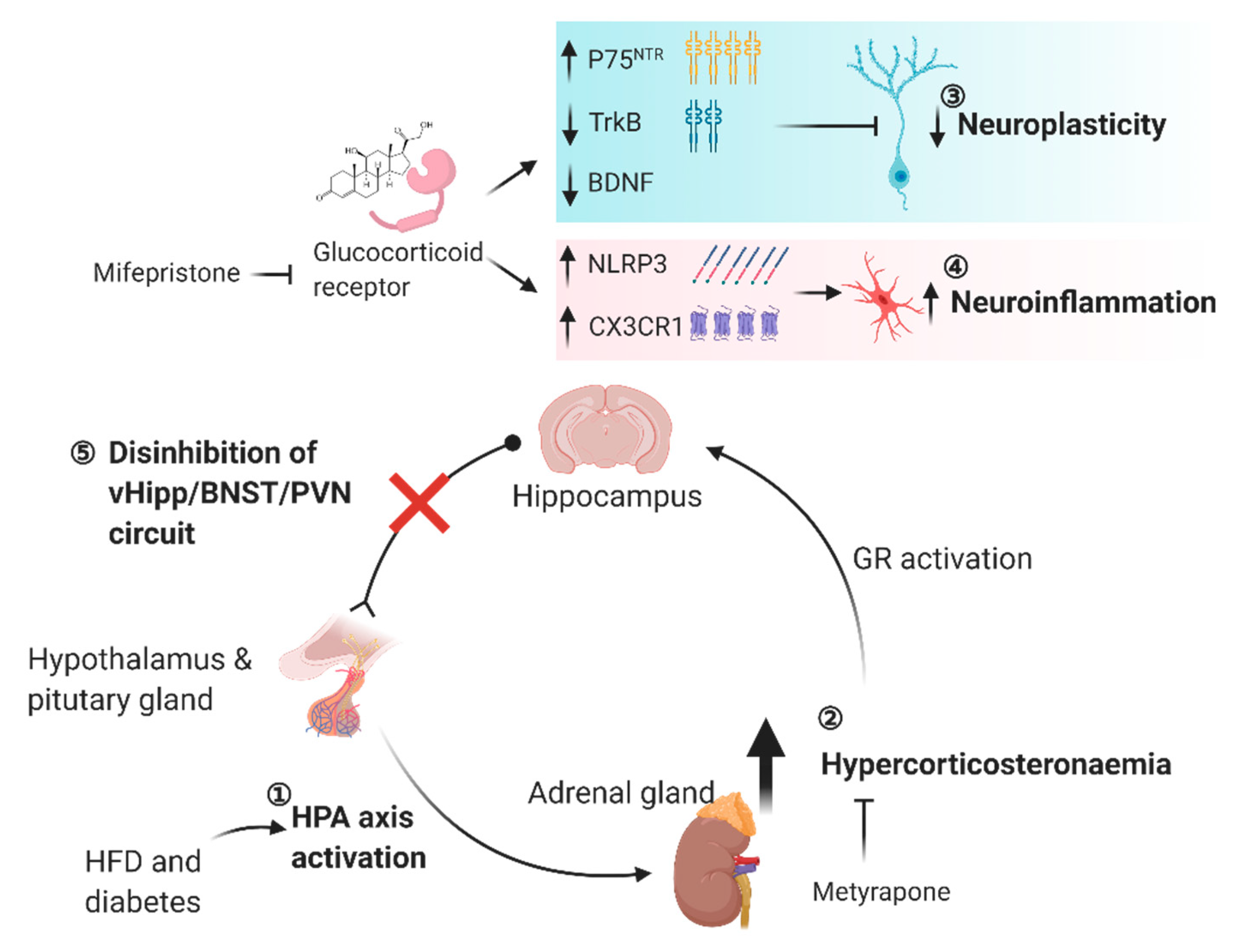
Figure 4. Cytokines secreted from peripheral tissue organs during exercise elicit pro-cognitive effects. The skeletal muscle secrets both lactate and irisin. In particular, both lactate and irisin can separately or concomitantly are engaged in modulating synaptic plasticity in the hippocampus. The adipose tissue secretes apelin, which reduces neuroinflammatory responses and promotes mitochondrial biogenesis in the neurons, respectively. The liver secretes β-hydroxybutyrate, a natural NLRP3 inhibitor, which can suppress the neuroinflammatory response. β-hydroxybutyrate can also promote neuroplasticity alongside with upregulating BDNF expression in the hippocampus. These exerkines are shown to be pro-cognitive by modulating both mood-related behaviours and learning and memory performance in the rodent studies. Created with BioRender.com.
Figure 4. Cytokines secreted from peripheral tissue organs during exercise elicit pro-cognitive effects. The skeletal muscle secrets both lactate and irisin. In particular, both lactate and irisin can separately or concomitantly are engaged in modulating synaptic plasticity in the hippocampus. The adipose tissue secretes apelin, which reduces neuroinflammatory responses and promotes mitochondrial biogenesis in the neurons, respectively. The liver secretes β-hydroxybutyrate, a natural NLRP3 inhibitor, which can suppress the neuroinflammatory response. β-hydroxybutyrate can also promote neuroplasticity alongside with upregulating BDNF expression in the hippocampus. These exerkines are shown to be pro-cognitive by modulating both mood-related behaviours and learning and memory performance in the rodent studies. Created with BioRender.com.

| Publisher's Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
5 Secret Health Benefits Of Gardening Working In Dirt Boosts Mood, Lowers Dementia Risk Kim Hayes
Source: https://www.mdpi.com/1422-0067/22/1/201/htm
Posted by: grandepoved1950.blogspot.com

0 Response to "5 Secret Health Benefits Of Gardening Working In Dirt Boosts Mood, Lowers Dementia Risk Kim Hayes"
Post a Comment